* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download CASE 35
Survey
Document related concepts
Transcript
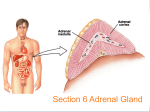
❖ CASE 35 A 36-year-old woman presents to her gynecologist with complaints of amenorrhea and hirsutism. She has also noticed an increase in her weight (especially in the trunk region) and easy fatigability. She denies any medical problems. Her periods were always normal until 6 months ago, and her hirsutism has been gradual in onset. On examination, she has a very rounded hirsute face with centripetal obesity. Her blood pressure is elevated, as is her weight compared with previous visits. On abdominal examination, she is noted to have striae and a male-like distribution of hair on the lower abdomen. The patient then undergoes studies that demonstrate increased cortisol production and failure to suppress cortisol secretion normally when dexamethasone is administered. She is diagnosed with Cushing syndrome. ◆ Where in the adrenal cortex is cortisol produced? ◆ How do glucocorticoids inhibit prostaglandin production? ◆ Why is hyperpigmentation not found in patients with secondary adrenocortical insufficiency? 286 CASE FILES: PHYSIOLOGY ANSWERS TO CASE 35: ADRENAL GLAND Summary: A 36-year-old woman with amenorrhea, hirsutism, elevated blood pressure, weight gain, insuppressible cortisol, and abdominal striae is diagnosed with Cushing syndrome. ◆ ◆ Cortisol production: Zona fasciculata. Glucocorticoids and prostaglandins: Inhibit cyclooxygenase and phospholipase A2, which are needed for prostaglandin formation. ◆ Secondary adrenocortical insufficiency and hyperpigmentation: There is a low level of adrenocorticotropic hormone (ACTH) with secondary adrenocortical insufficiency. ACTH contains the MSH fragment, and when it is elevated (primary adrenocortical insufficiency), hyperpigmentation may occur. CLINICAL CORRELATION Cushing syndrome is a condition caused by increased cortisol production. This increase may be the result of overproduction of ACTH (Cushing disease) or excess production of cortisol by the adrenal gland (adrenal hyperplasia). A patient’s symptoms may include truncal obesity, a round “moon” face, a “buffalo hump,” hypertension, fatigability and weakness, amenorrhea, hirsutism, abdominal striae, osteoporosis, and hyperglycemia. The diagnosis is presumed when there is increased cortisol production and failure to suppress cortisol secretion with dexamethasone. The level of ACTH will help differentiate whether the problem is from overproduction of ACTH or from increased adrenal cortisol production. Treatment depends on the etiology. Overproduction from an ACTH-secreting tumor usually requires surgical intervention. Medical management of adrenal hyperplasia may include the use of ketoconazole or other medications that inhibit steroidogenesis. APPROACH TO ADRENAL PHYSIOLOGY Objectives 1. 2. 3. Understand the structure of the adrenal cortex and medulla. Know about regulation of adrenocortical hormones. Understand the actions of adrenocortical hormones. Definitions 1. Glucocorticoids: Steroid hormones, primarily cortisol, secreted by the adrenal cortex that promote the synthesis of enzymes involved in energy balance and fuel utilization. They have potent immunosuppressant and CLINICAL CASES 2. 3. 287 anti-inflammatory activities and their secretion is increased in response to stress. Mineralocorticoids: Steroid hormones, primarily aldosterone, that are secreted by the adrenal cortex and are essential for the maintenance of salt and water balance by the kidney. ACTH: Adrenocorticotrophic hormone is secreted by the anterior pituitary gland and directly controls the rate of production of the glucocorticoids and androgens by the adrenal cortex. DISCUSSION The suprarenal, or adrenal, glands are a pair of structures just above the kidneys. The adrenals form a complex structure consisting of functionally and morphologically distinct regions: an outer region, the adrenal cortex, and the inner medulla. The adrenal cortex is the site of synthesis and secretion of steroid hormones known as the mineralocorticoids, the glucocorticoids, and the androgens. The bulk of the adrenal gland is the cortex, which is composed of morphologically and functionally distinct segments. Descending into the gland just below the capsule is the zona glomerulosa, clusters of cells that secrete the mineralocorticoid aldosterone. The zona fasciculata penetrates deeply into the cortex and overlays the zona reticularis. These two regions produce the glucocorticoids and the adrenal androgens. The innermost region is the medulla. The adrenal medulla is heavily innervated and is a source of the circulating sympathetic hormones epinephrine and norepinephrine. Regulation of Adrenal Cortical Hormone Secretion The rate of secretion of the adrenal cortical hormones is dependent on their rate of production. Unlike the peptide hormones, for example, the steroid hormones are permeable to the plasma membranes of cells, and their concentration in the cell is the determinant of the rate at which they leave the cell and enter the plasma. In the plasma, they bind to and are transported by globular proteins such as corticosteroid-binding protein and albumin. The adrenal cortical hormones act on target tissues by diffusing into the cell and forming a complex with a specific intracellular receptor. Complex formation exposes a DNA-binding site and is translocated into the nucleus. In the nucleus, the hormone–receptor complex binds to a specific site on the DNA target molecule, the hormone responsive element, and enhances or inhibits gene transcription. The production of glucocorticoids and androgens is directly controlled by ACTH. ACTH binds to a receptor on the cell surface which mediates the activation of the enzymes involved in the synthesis of pregnenolone, the precursor molecule of adrenocortical hormones produced in the zona fasciculata and the zona reticularis. ACTH actions result in the direct activation of enzymatic activities and a slower increase in the synthesis of these enzymes. Cortisol secretion normally follows that of ACTH, with a diurnal pattern of secretion 288 CASE FILES: PHYSIOLOGY peaking in the early morning hours. ACTH is produced in the anterior lobe of the pituitary gland in response to hypothalamic release of corticotropinreleasing hormone (CRH) from the paraventricular nuclei. The circulating level of cortisol is regulated by a negative feedback control of ACTH secretion. Cortisol inhibits ACTH secretion by acting directly on the ACTHproducing cells and indirectly by inhibition of the hypothalamic neuronal release of CRH. The mechanism of cortisol inhibition involves cortisol binding to corticosteroid receptors in these tissues and inhibition of specific gene transcription. Cushing syndrome is often the result of increased pituitary secretion of ACTH because of a pituitary adenoma. There are however other types of tumors (outside the pituitary) that secrete ACTH and stimulate cortisol production. To help identify the cause of the elevated cortisol a test is conducted with the administration of dexamethasone, a synthetic analog of cortisol, to inhibit ACTH release and thus cortisol production. The failure to lower cortisol production indicates a nonpituitary source of the problem. Physiology of Glucocorticoids Cortisol has a permissive effect on a number of enzymes that mediate the breakdown of fats and protein and hepatic glucose production and is essential for the maintenance of energy balance and fuel utilization in the body. Cortisol also has potent anti-inflammatory and immunosuppressant activities that make it an extremely important therapeutic agent. The glucocorticoids, primarily cortisol and to a lesser extent corticosterone, play a central role in the physiologic response to stress, and their secretion is increased during stress. Factors such as surgery, hypoglycemia, and pain stimulate ACTH secretion and consequently stimulate cortisol production and secretion. Severely stressful conditions will override both diurnal ACTH production and the feedback inhibition of cortisol on ACTH secretion, resulting in a sustained ACTH level and maximal levels of cortisol production. The physiology of the glucocorticoids is complex and can influence and alter the function of every system in the body. The dominant and most crucial functions involve energy metabolism through the control of the expression of proteins that are essential for the maintenance of energy balance during periods of food deprivation. During periods of fasting, the body rapidly exhausts its carbohydrate supplies and depends on other sources of fuel for metabolic energy. In the liver, cortisol increases the synthesis of enzymes in the gluconeogenic pathway, as well as enzymes in muscle tissue for the breakdown of proteins to provide glucogenic precursors for glucose synthesis. Glucocorticoiddependent enzymes catalyze the breakdown of fats through lipolysis to generate free fatty acids and keto acids for energy production. The combined effect of these actions is to decrease glucose utilization by providing alternate fuels and to maintain plasma glucose levels for tissues that have an absolute requirement for glucose as oxidizable substrate. CLINICAL CASES 289 Corticosteroids have potent anti-inflammatory activity. Tissue injury or other stimuli lead to an increased production of the arachidonic acid derivatives, prostaglandins and leukotrienes. The injury activates a phospholipase (PLA2) that releases arachidonic acid from phospholipids. The newly formed arachidonic acid then is converted to the prostaglandin structure by a cyclooxygenase. Physiologically, prostaglandins have localized effects of dilating the vessels in the area of the injury and sensitizing the nerve endings and increasing the sensation of pain. Glucocorticoids are also negative regulators of cytokine production, particularly interleukin-1 (IL-1) and tumor necrosis factor-a (TNF-a) and numerous other mediators. These cytokines are produced primarily in macrophages after stimulation by immune complexes and arachidonic acid metabolites and after injury. In addition to inhibiting their production, the steps outlined above identify loci where glucocorticoids interfere with the signaling pathways and the actions of their products. IL-1 production by macrophages plays a central role in the immune response; thus, glucocorticoids are immunosuppressive. The glucocorticoids are effective immunosuppressive agents and are an important therapeutic tool. Proliferation of β-lymphocytes requires cytokines released from helper T cells. Glucocorticoids inhibit macrophage and T-cell cytokine production, which reduces B-cell proliferation. T-cell proliferation is induced by IL-2 after exposure to an antigen. Glucocorticoids inhibit IL-2 production, thereby reducing T-cell proliferation. By these mechanisms, glucocorticoids are able to suppress both humeral and cellular immune responses. The Physiology of Mineralocorticoids The mineralocorticoids, primarily aldosterone, are essential for the maintenance of salt and water balance by the kidney by regulating sodium reabsorption and potassium secretion. Aldosterone is produced in the zona glomerulosa from the common pregnenolone precursor. Regulation of aldosterone secretion is dependent on several factors. ACTH-dependent pregnenolone synthesis is required for maximal aldosterone production, but secretion is controlled directly by angiotensin II and the extracellular ([K+]) potassium concentration. Increasing plasma [K+] stimulates aldosterone secretion. Atrial natriuretic factor has a negative modulatory effect on aldosterone secretion. As with the other adrenal cortical hormones, aldosterone binds to and is carried in the blood by corticosteroid-binding globulin (CBG). The physiologic role of aldosterone is the maintenance of fluid and electrolyte balance, and its secretion is tightly coupled to the vascular volume. A decrease in vascular volume initiates a cascade response with an increase in renal renin secretion from smooth muscle cells of the afferent glomerular arteriole. Renin catalyzes the conversion of angiotensinogen to angiotensin I. Angiotensin-converting enzyme (ACE) converts angiotensin I to angiotensin II. The target of angiotensin II is the zona glomerulosa, which responds by increasing the production and secretion of aldosterone. 290 CASE FILES: PHYSIOLOGY COMPREHENSION QUESTIONS [35.1] Dexamethasone is a synthetic analogue of cortisol. Therapeutically, it can be used to block the effects of conditions with excessive cortisol secretion. Which of the following is the best description of the mechanism of action of dexamethasone? A. B. C. D. [35.2] Mineralocorticoids play a critical role in fluid and electrolyte balance in the body. A normal response to aldosterone is to increase acid secretion in the kidney. The hyperaldosteronism that occurs with some adrenal tumors has interesting and profound effects on acid–base balance as a result of increased renal H+ secretion. Which of the following would be the most likely result of hyperaldosteronism? A. B. C. D. E. [35.3] Binds to cortisol Binds to the adrenal gland Competes for cortisol-binding sites Inhibits ACTH secretion Excretion of excess bicarbonate Generation of metabolic alkalosis Hyperkalemia caused by renal K+ resorption Increased H+ resorption by renal tubular cells Movement of K+ out of cells in exchange for H+ Adrenalectomized animals are unable to survive even brief periods of food deprivation. Cortisol replacement restores the ability to survive a fast. Which of the following is the best explanation for this observation? A. Cortisol can act synergistically with insulin. B. Cortisol can act antagonistically to the action of growth hormone. C. Cortisol has a permissive effect on enzymes involved in the mobilization of various fuels. D. Cortisol increases glucose utilization and decreases glycogen storage. Answers [35.1] D. Cortisol production and secretion are controlled by ACTH, which is secreted from the anterior lobe of the pituitary gland. A feedback inhibition loop allows cortisol to inhibit ACTH secretion. The synthetic glucocorticoid dexamethasone effectively and abruptly blocks ACTH secretion, thereby suppressing cortisol secretion. [35.2] B. Hyperaldosteronism leads to a loss of potassium and hypokalemia. The hypokalemia leads to a loss of potassium from cells largely in exchange for H+. This causes a metabolic alkalosis and an intracellular acidosis. Renal tubular cells respond with an increase in the rate of H+ secretion. Paradoxically, despite the metabolic alkalosis, there CLINICAL CASES 291 is an increased rate of H+ secretion and bicarbonate reabsorption with the addition of bicarbonate to the blood. There are increased reabsorption of bicarbonate and maintenance of the metabolic alkalosis. [35.3] C. Cortisol is essential for the expression of numerous enzymes involved in the maintenance of fuel supplies in preparation for and during a fast. At each stage of metabolism, enzymes have been identified as cortisol-dependent, beginning with the generation of glucogenic precursors. Muscle protein catabolism is an essential source of carbohydrate precursors. Cortisol increases proteolysis and induces the transaminases necessary to convert pyruvate into alanine for transport to the liver for gluconeogenesis. Key enzymes in the gluconeogenic pathway ranging from pyruvate to glycogen, and those involved in the release of glucose from the liver are all known to be inducible by cortisol. Thus, one of its main functions is the continued production of glucose for metabolism by glucose-dependent tissues during a fast. Glucose utilization by tissues that can utilize alternative fuels is reduced by cortisol, which antagonizes insulin-dependent processes. To provide the energy necessary for gluconeogenesis, cortisol enhances triglyceride breakdown and free fatty acid mobilization from fat stores. In addition, cortisol acts synergistically with glucagon to enhance hepatic gluconeogenesis. PHYSIOLOGY PEARLS ❖ ❖ ❖ ❖ ❖ Adrenal glands have an outer cortical region that produces the mineralocorticoids (aldosterone), glucocorticoids (cortisol), and androgenic hormones. The inner, medullary region of the adrenal gland is highly innervated and produces the circulating sympathetic hormones epinephrine and norepinephrine. The adrenocortical hormones are steroid hormones. The steroid hormones are permeable to the cell membrane; thus, their rate of secretion is dependent on their rate of production. Cortisol production is dependent on ACTH and in part is controlled by a negative feedback loop with cortisol-inhibiting ACTH production. In the present case, there is an increase in ACTH secretion that causes an overproduction of cortisol (Cushing syndrome). Adrenal hyperplasia also can cause overproduction of cortisol. Understanding the mechanisms of action of the adrenocortical hormones has produced a number of novel therapies for the control of hypertension (ACE inhibitors), anti-inflammatories (COX 2 inhibitors), and immunosuppression (cortisol). 292 CASE FILES: PHYSIOLOGY REFERENCES Genuth SM. The adrenal glands. In: Berne RM, Levy MN, eds. Physiology. 4th ed. St. Louis, MO: Mosby; 1998:930-964. Goodman HM. Adrenal glands. In: Johnson LR, ed. Essential Medical Physiology. 3rd ed. San Diego, CA: Elsevier Academic Press; 2004:607-635.