* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download BE REU @ SLU Department of Chemistry Dr. Shelley D. Minteer
Survey
Document related concepts
Transcript
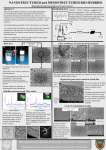
Enzymatic Glucose Biofuel Cell: Concentration Studies and Biocompatibility Joe Fazio BE REU @ SLU Dr. Shelley D. Minteer Kyle Sjöholm SLU Department of Chemistry Background Enzymatic Biofuel cell: Enzymes Power biomedical devices High power and current density Incomplete oxidation www.nano-biokit.com Biofuel Cell Process Reaction at anode produces protons Electrons create current Protons diffuse to cathode Protons at cathode react with oxygen Mediated Electron Transfer (MET) Electrocatalyst Commonly used to reduce overpotential 2e- Glucose NADH Facilitates ion transfer to electrode Gluconolactone NAD+ Glucose Dehydrogenase Entrapped in Polymer 060 Toray Paper Electrode Modified Polymers Immobilize enzymes Extend functional lifetime Microencapsulation: Support enzyme structure Neutral pH Micellar environment Geometry Ion exchange properties Polymer encapsulation Project Goals Power Densities Hypoglycemic (3mM) Normal (5mM) Hyperglycemic (8mM) Biocompatibility Bulk electrolysis Live/dead assay • Biofilm formation Basic Components Anode: 060 Toray Paper electrodes Fuel: Glucose Enzyme: Glucose Dehydrogenase Cofactor: NAD+ Electrocatalyst: Poly(methylene green) (PMG) Modified polymer: Nafion® Chitosan Electrode Preparation Polymer modification Nafion®: Tetrabutylammonium bromide (TBAB) Chitosan Hydrophobic Deacylation Chitosan http://www.global-b2b-network.com/ Co-cast polymer and enzyme onto electrode Soak electrodes in solution of glucose overnight Experimental Set-up 3, 5, 8mM glucose fuel NAD+, pH 7.4 phosphate buffer Bioanode V Glass tube Fuel Solution Open circuit potential (~1000secs) Linear sweep voltammetry (<1mV/sec) Power density equation P=I*V + O-ring Nafion PEM 4.5cm 2 20% Pt GDE Cathode O-ring Glass tube Diagram of Icell Air Potentiostat Power Density Test Results 5mM Averages 7e-6 7e-6 6e-6 6e-6 Power Density, Watts/cm2 Power Density, Watts/cm2 3mM Averages 5e-6 4e-6 3e-6 2e-6 1e-6 4e-6 3e-6 2e-6 1e-6 0 0 0 1e-5 2e-5 3e-5 Current Density, Amps/cm 4e-5 2 0 1e-5 Deacylated Chitosan Chitosan Nafion 8mM Averages 8e-6 Power Density, Watts/cm2 5e-6 2e-5 3e-5 Current Density, Amps/cm 4e-5 5e-5 2 Average Maximum Power Density* µW/cm2 6e-6 4e-6 2e-6 0 0 1e-5 2e-5 3e-5 Current Density, Amps/cm2 4e-5 5e-5 3mM 5mM 8mM Chitosan 2.87(±0.21) 2.82(±0.52) 3.32(±0.46) Deacylated chitosan 6.04(±3.23) 6.15(±3.51) 7.52(±4.31) Nafion® 0.28(±0.02) 0.29(±0.02) 0.33(±0.04) *errors are equal to one standard deviation Biocompatibility, Bulk Electrolysis Decreasing current Possible biofilm formation 6e-6 5e-6 Current, Amps Testing Bacteria culture injected Hold fuel cell at 0.3V and monitor current (3 days) 4e-6 3e-6 2e-6 1e-6 0 0.0 5.0e+4 1.0e+5 1.5e+5 Time, seconds 2.0e+5 2.5e+5 Biocompatibility, Live/dead Assay Live/Dead assay Cast polymer with bacteria Gluconobacter SP33 Origami C4-AW genetically modified E. Coli Fluorescent nucleic acid stains FITC filter- live bacteria TRITC filter- dead bacteria Live/Dead Assay Assay showed biocompatibility for all polymers. FITC filter Chitosan E. coli Deacylated chitosan Gluconobacter Nafion® E. coli Nafion® Gluconobacter TRITC filter image Olympus IX71 fluorescence microscope Conclusions Chitosan and Nafion® can immobilize GDH Chitosan provides higher power and current densities Chitosan and Nafion® provide biocompatible surface material Future work Temperature and pH studies Biocompatible modifications Impact on current densities Acknowledgements National Science Foundation Saint Louis University Dr. Minteer Minteer group Kyle Sjöholm Dr. Waheed Rob Arechederra 1) 2) 3) 4) 5) 6) 7) 8) 9) 10) 11) 12) 13) 14) 15) 16) 17) 18) 19) 20) 21) 22) 23) 24) 25) References Akers, Moore, Minteer. “Development of Alcohol/O2 Biofuel Cells Using Salt-Extracted Tetrabutylammonium Bromide/Nafion Membranes to Immobilize Dehydrogenase Enzymes.” Electrochimica Acta 50 (2005): 2521-2525. Arechederra, Robert, Shelley D. Minteer. “Organelle-based Biofuel Cells: Immobilized Mitochondria on Carbon Paper Electrodes.” Electrochimica Acta 53 (2008): 6698-6703. Atanassov, Plamen, et al. “Enzymatic Biofuel Cells. The Electrochemical Society Interface (2007). Beilke, Michael C., et al. “Enzymatic Biofuel Cells.” Micro Fuel Cells Principles and applications. T.S. Zhao. Publisher location: Elsevier, 2009. 179-242. print. Blackwell, Anne E, et al. “Comparison of Electropolymerized Thiazine Dyes as an Electrocatalyst in Enzymatic Biofuel Cells and Self Powered Sensors.” Nanoscience and Nanotechnology 9.3 (2009): 1714-21. Bond, Alan M, et al. “A Role for Electrospray Mass Spectrometry in Electrochemical Studies.” Analytical Chemistry 67(1995):1691-1695. Cooney, M.J., et al. “Enzyme Catalysed Biofuel Cells.” Energy & Environmental Science 1 (2008): 320-337. Cox, James A., Thomas J. Gray, “Controlled-Potential Electrolysis of Bulk Solutions at a Modified Electrode: Application to Oxidations of Cysteine, Cystine, Methionine, and Thiocyanate.” Analytical Chemistry 62(1990): 2742-2744. Crittenden, Scott R., Christian J. Sund, James J. Sumner. “Mediating Electron Transfer from Bacteria to a Gold Electrode via a Self-Assembled Monolayer.” Langmuir 22(2006):9473-9476. Galassetti, Pietro R., et al. “Breath Ethanol and Acetone as Indicators of Serum Glucose Levels: An Initial Report.” Diabetes Technology & Therapeutics 7(2005):115123. Hu, Qiang, A. Scott Hinman. “A Bulk Electrolysis Raman Spectroelectrochemical Cell Using a Rotating Electrode.” Analytical Chemistry 72(2000): 3233-3235. Ikeda, Tokuji. “A Novel Electrochemical Approach to the Characterization of Exidoreductase Reactions.” The Chemical Record 4(2004):192-203. Klotzbach, Tamara L., Michelle Watt, Yasmin Ansari, Shelley D. Minteer. “Improving the microenvironment for enzyme immobilization at electrodes by hydrophobically modifying chitosan and Nafion® polymers.” Journal of Membrane Science 311(2008):81-88. “Live/Dead Baclight Bacteria Viability Kitis.” Molecular Probes Inc. 2004. Mano, Nicolas, “A 280µW cm-2 biofuel cell operating at low glucose concentration.” The Royal Society of Chemistry (2008):2221-2223. Martin, Georgianna L., Shelley D. Minteer, Michael J. Cooney. “Spatial Distribution of Malate Dehydrogenase in Chitosan Scaffolds.” Applied Materials & Interfaces 1 (2009):367-372. Minteer, Shelley D., Bor Yann Liaw, Michael J. Cooney. “Enzyme-based biofuel cells.” Current Opinion in Biotechnology 18 (2007):228-234. Moore, Christine M, et al. “Improving the Environment for Immobilized Dehydrogenase Enzymes by Modifying Nafion with Tetraalkylammonium Bromides.” Biomacromolecules 5 (2004): 1241-1247. Subramanyam, Elango, Sidharthan Mohandoss, Hyun-Woung Shin. “Synthesis, Characterization, and Evaluation of Antifouling Polymers of 4Acryloyloxybenzaldehyde with Methyl Methacrylate.” Journal of Applied Polymer Science 112(2009):2741-2749. Tamaki, T., T. Ito, T. Yamaguchi. “Modelling of Reaction and Diffusion Processes in a High-surface-area Biofuel Cell Electrode Made of Redox Poluymer-grated Carbon.” Fuel Cells 09 1(2009):37-43. Wang, J., et al. “The effects of amorphous carbon films deposited on polyethylene terephthalate on bacterial adhesion.” Biomaterials 25(2004):3163-3170. Heikkila, O., N Lundbom, M Timonen, P-H Groop, S Heikkinen, S Makimattila. “Hyperglycaemia is associated with changes in the regional concentrations of glucose and myo-inositol within the brain.” Diabetologia 52(2009):534-540. Gupta, Sandeep, Eugene Chough, Jennifer Daley, Peter Oates, Keith Tornheim, Neil B. Ruderman, and John F. Keaney Jr. “Hyperglycemia increases endothelial superoxide that impairs smooth muscle cell Na+-K+-ATPase activity.” Am J Physiol Cell Physiol 282(2002): C560-C566. Mason RM,Thomas G, Davies M. “Proteoglycan synthesis by human mesangial cells is depressed by hyperglycemic glucose concentrations.” Biochemical society transactions 2(1992): 96 Xiaoli, Ma, Yao Zihua, Shi Dagang. “Preparation and characterization of porous chitosan membranes and the localization of the activity of urease immobilized on it by SEM and X-ray microanalysis.” Chemical Journal on Internet 7(2005): 45.