* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download Direct and indirect diagnostic methods in
Hospital-acquired infection wikipedia , lookup
Globalization and disease wikipedia , lookup
History of virology wikipedia , lookup
Neonatal infection wikipedia , lookup
Transmission (medicine) wikipedia , lookup
Germ theory of disease wikipedia , lookup
Community fingerprinting wikipedia , lookup
Infection control wikipedia , lookup
Marine microorganism wikipedia , lookup
Surround optical-fiber immunoassay wikipedia , lookup
Human cytomegalovirus wikipedia , lookup
Virus quantification wikipedia , lookup
Molecular mimicry wikipedia , lookup
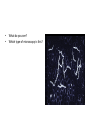
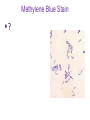
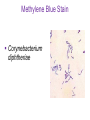
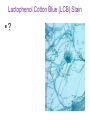
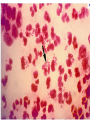
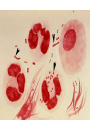
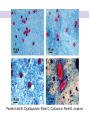
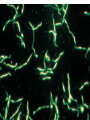
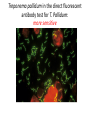
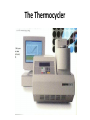
Direct and indirect diagnostic methods in infectious diseases Learning Objectives At the end of this lecture, the student should be able to: • list the main methods in diagnosis of different type of microorganisms • explain the importance of these methods in diagnosis • List the main advantages and disadvantages of each type of test Case I • 6 year old child presented with a 24 hour history of fever, vomiting and complaint of a sore throat Etiology • Strep throat: S. Pyogenes (A group Beta hemolytic streptococci) – Mainly viruses – Rarely: • Corynebacterium • Arcanobacterium • Neisseria gonorrhoeae Laboratory Diagnosis Diagnosis • Rapid antigen test • Culture and susceptibility test ? Case II • Fever • History Case II • Fever • Has a history to visit to west Africa • Ebola • Malaria Case • • • • • • Peripheral blood smear: Plasmodium Babesia Trypanosome Microfilaria Bacteria and fungi Case • 1.5 years old patient presenting to the emergency room with 2 hour of history of vomitting, diarrhea, fever • Stool macroscopy: no mucus no blood Stool microscopy • Fecal leucocyte • Culture: Salmonella, Shigella and camphylobacter Fecal leucocyte Fecal leucocyte absent Season • Winter For rapid diagnosis • Antigen detection – Rotavirus Outbreak! • Norovirus antigen Multiplex PCR case • A child • Maculupapular rash case • Maculupapular rash • Viral(measles, rubella, HHV6,parvovirus B19,EBV, Enterovirus,CMV • Others • HIV Laboratory Diagnosis Maculopapular rash • • • • • Specific serology: IgM Total antibody Antigen and antibody NAT Laboratory diagnosis • Direct • Indirect Laboratory diagnosis • Direct: -Microscopy -Culture -Antigen -Nucleic acid • Indirect: -Specific antibody (Serology) Laboratory diagnosis • Direct: -Microscopy -Culture -Antigen -Nucleic acid • Indirect: -Specific antibody (IgG, IgM, IgA) Laboratory diagnosis • Direct: -Microscopy -Culture -Antigen detection -Nucleic acid detection: Nucleic acid amplification techniques (NAT=NAAT) • Indirect: -Specific antibody (IgG, IgM, IgA) • What do you see? • Which type of microscopy is this? Microscopic Principles and Applications • In general, microscopy is used in microbiology for two basic purposes: 1-the initial detection of microbes 2-the preliminary or definitive identification of microbes. Microscopic Principles and Applications • The microscopic examination of clinical specimens is used to detect: - bacterial cells, - fungal elements, - parasites (eggs, larvae, or adult forms), and - viral inclusions present in infected cells. - But lacks sensitivity ! Stains Because most organisms are colorless and transparent, various dyes (stains) are used to see the individual cells A variety of different types of stains are used in the microbiology lab, including: Contrast stains (e.g., methylene blue, lactophenol cotton blue, India ink, iodine) Differential stains (e.g., Gram stain, spore stains, acid-fast stains, Giemsa stain, silver stains, Trichrome stain) Fluorescent stains (e.g., acridine orange, auramine-rhodamine, calcofluor white, antibody-conjugated fluorescent stains) 32 Methylene Blue Stain ? 33 Methylene Blue Stain Corynebacterium diphtheriae 34 Direct Examination The sample: • can be mixed with alkali to dissolve background material (potassium hydroxide [KOH] method) : fungal elements • mixed with a combination of alkali and a contrasting dye (e.g., lactophenol cotton blue: fungal elements Lugol iodine :parasitology specimen Lactophenol Cotton Blue (LCB) Stain ? 36 Lactophenol Cotton Blue (LCB) Stain primarily for observing the morphology of fungal molds : Aspergillus 37 Enterobius vermicularis Pinworm eggs: deposited by adults at night in the perianal area. Eggs are collected by pressing tape on the anal surface Eggs appear as an embryo surrounded by a colorless shell that is characteristically flattened on one side. 38 Iodine Stain ? 39 Iodine Stain The iodine stain is a contrast stain used primarily for the detection of intestinal parasites (Entamoeba coli in this example). 40 Direct Examination India ink method, • in which the ink darkens the background rather than the cell. • This method is used to detect capsules surrounding organisms, such as the yeast Cryptococcus (the dye is excluded by the capsule, creating a clear halo around the yeast cell), and • is a rapid method for the preliminary detection and identification of this important fungus. • India Ink Stain ? 42 India Ink Stain The India ink stain: negative contrasting stain Cryptococcus neoformans. The ink is excluded by the fungal capsule so the fungi (arrows) are unstained and surrounded by a clear halo, while the ink particles provide a background contrast. But now antigen detection is preferred 43 Differential Stains • Gram stain : -bacteria -Yeasts (yeasts are grampositive). Gram staining • Neisseria gonorrhoae detection in uretral specimen from males Differential Stains • Acid-Fast Stains • Ziehl-Neelsen stain: Used to stain mycobacteria and other acid-fast organisms. • Kinyoun stain: Cold acid-fast stain (does not require heating) Updated Guidelines for the Use of Nucleic Acid Amplification Tests in the Diagnosis of Tuberculosis Conventional tests for laboratory confirmation of TB include • acid-fast bacilli (AFB) smear microscopy(24 hours) • culture Although rapid and inexpensive, AFB smear microscopy is limited by its poor sensitivity (45%–80% with culture-confirmed pulmonary TB cases) Acid-Fast Stains 51 Acid-Fast Stains Mycobacteria If a weak decolorizing solution is used to remove the primary stain, then partially acid-fast organisms such as Nocardia 52 • Auramine-rhodamine: Same principle as other acid-fast stains, except that fluorescent dyes (auramine and rhodamine) are used for primary stain • Modified acid-fast stain: Weak decolorizing agent is used with any of three acid-fast stains listed. Whereas mycobacteria are strongly acid-fast, other organisms stain weaker (e.g., Nocardia, Rhodococcus, Tsukamurella, Gordonia, Cryptosporidium, Isospora, Sarcocystis, and Cyclospora). • These organisms can be stained more efficiently by using weak decolorizing agent. Organisms that retain this stain are referred to as partially acid-fast. Panels A and B, Cryptosporidia. Panel C, Cyclospora. Panel D, Isospora. 54 Differential Stains • Giemsa stain: blood parasites Giemsa Stain differential stain used for detection of parasites in blood smears 56 Giemsa Stain Plasmodium 57 Darkfield Microscopy • Treponema pallidum (syphilis): ! used in routine diagnosis • Leptospira spp. (leptospirosis) not used Microscopic Principles and Applications • The microscopic detection of organisms stained with antibodies labeled with fluorescent dyes or other markers has proved to be very useful for the specific identification of many organisms. Treponema pallidum in the direct fluorescent antibody test for T. Pallidum: more sensitive In Vitro Culture: Principles and Applications • Anton van Leeuwenhoek : Microscobic observation (1676 ) • Pasteur: culture of bacteria almost 200 years later • Over the years, microbiologists and cooks have returned to the kitchen to create hundreds of culture media that are now routinely used in all clinical microbiology laboratories. In Vitro Culture: Principles and Applications • Although tests that rapidly detect microbial antigens and nucleic-acid-based molecular assays have replaced culture methods for the detection of many organisms, • the ability to grow microbes in the laboratory remains an important procedure in all clinical labs. • For many diseases, the ability to grow a specific organism from the site of infection is the definitive method to identify the cause of the infection. • Antibiotic susceptibility test The success of culture methods is defined by: • the biology of the organism • the site of the infection • the patient's immune response to the infection • the quality of the culture media. Certain bacteria need special conditions: • Legionella is an important respiratory pathogen; media should be supplemented with iron and lcysteine. • Campylobacter, an important enteric pathogen, highly selective media should be incubated at 42° C in a microaerophilic atmosphere. • Chlamydia, an important bacterium responsible for sexually transmitted diseases, is an obligate intracellular pathogen that must be grown in living cells. Types of Culture Media Culture media can be subdivided into four general categories: (1) enriched nonselective media, (2) selective media, (3) differential media, and (4) specialized media Cell Culture: not routine ! • Some bacteria and all viruses are strict intracellular microbes • They can only grow in living cells. • In 1949, Enders described a technique for cultivating mammalian cells for the isolation of poliovirus. • This technique has been expanded for the growth of most strict intracellular organisms. Serologic Methods (Immunologic techniques) • Detect • Identify • Quantitate antigen or antibody Disadvantage: Cross reaction: False positivity -similar or common epitope Serologic, Serodiagnosis, Serology • Detection of antigen or antibody in serum • The term serologic is used also for searching antigen or antibody in mediums other than serum(saliva,urine) • Serologic assay=immunoassay Immunoassays • Antigen or antibody is detected • In a variety of clinical specimens: – Mostly sera – Body fluids(cerebrospinal fluid) – Tissues – Environmental substances Antibodies Polyclonal: • Heterogeneous antibody preparations • Recognizes many epitopes on a single antigen Monoclonal: • Recognize individual epitoses on an antigen Methods of detection Antibody-antigen complexes can be detected: • Directly • Labelling the antibody or the antigen: -enzyme -radioactive -fluorescent dye Classical serologic methods • Precipitation • Immunodiffusion techniques • Agglutination Other serologic methods • Complement fixation • Hemagglutination inhibition • Neutralization Agglutination tests • Clumping of antigen with its antibody • Flocculation: similar to agglutination; except that agglutinats float rather than sediment • Prozone reaction: high antibody causes false negative. The sera should be diluted!! • Antigens passively absorbed on carriers:passive agglutination Agglutination tests • Antigens passively absorbed on carriers:passive agglutination -Red blood cells: passive hemagglutination -gelatin particles: particle agglutination Classical agglutination in test tubes: -Salmonella:Gruber Widal -Brucella:Wright -Rickettsiae:Weil-Felix reaction Agglutination negative Agglutination positive Immunoassays • Immunofluorescence (IFA) • Enzyme-linked immunosorbant assay (ELISA) -Western blot • Radioimmunoassay (RIA) Serology • can be used to identify the infecting agent • evaluate the course of an infection, or determine the nature of the infection-whether it is a primary infection or a reinfection, and whether it is acute or chronic. • Serologic testing is used to identify viruses and other agents that are difficult to isolate and grow in the laboratory or that cause diseases that progress slowly In the diagnosis of infectious diseases by immunoassays • Either spesific antigen: – Directly from specimen – From the culture for identification • Specific antibodies are detected: – IgG – IgM – IgA Specific antibody detection • Seroconversion occurs when antibody is produced in response to a primary infection. • IgM: early in infection (2-3 weeks) transient (3-6 months) *sometimes persists longer • IgG: forms later (immunity) highest in 4-6 months usually persists during the whole • IgG avidity: High: past infection Low: new infection life Examples of Viruses Diagnosed by Serology • • • • • • Epstein-Barr virus Rubella virus, Measles,Mumps Hepatitis A, B, C, D, and E viruses Human immunodeficiency virus Human T-cell leukemia virus Arboviruses (encephalitis viruses) Diagnosis of acute infection • • • • • • • By specific IgM detection by ELISA: HAV Measles Rubella Mumps Parvovirus B19 Varicella zoster… Quantitative antibody detection: • Anti-HBs: 10mIU/ml(immune to HBV infection) • Rubella IgG: 10-15 IU/ml(immune to Rubella infection) Antigen detection • • • • • • Membrane ELISA Immunochromatograhic methods Latex agglutination Rapid Less sensitive Less specific Antigen detection • • • • • • • Strep A RSV Influenza Rota Norovirus Legionella pneumophila serogroup I Malaria Nucleic acid amplification techniques (NAT=NAAT) • Target molecule – DNA – RNA Molecular Diagnosis • • • • The advantages of molecular techniques: their sensitivity Specificity safety.. P C R olymerase hain eaction Requirements of PCR: • Knowing parts of the target DNA sequence to be amplified • Two types of synthetic primers, complementary to the ends of the target sequence • Large amounts of the four DNA nucleotides • Taq1, a heat-resistant form of DNA Polymerase How it works… Number of amplified pieces = 2n (n = # of cycles) The Thermocycler PCR • The polymerase chain reaction (PCR): • amplifies single copies of viral DNA millions of times over • Very sensitive Danger !: • Contamination • False positive Postamplification detection • Gel analysis • Colorimetric microtitre plate system • Target amplification and detection systems occur simultaneously in the same tube (RealTime PCR): -sensitive -specific -rapid -easy to use -expensive RV12 İnfluenza A Rapid molecular techniques!!! Rapid real –time PCR Direct sequencing • Combination of PCR with dideoxynucleotide chain termination methods can be used to determine sequence of DNA. • Genotyping of viruses • Identification of bacteria and fungi • Antimicrobial susceptibilty testing to detect mutations DNA microarrays • Thousands of oligonucleotides are on a solid support • A labelled amplification product is hybridized to the probes Microarray Multiplex PCR: Now more often: !!!! -antigen detection -rapid real-time PCR -multiplex PCR -quantitative detection for followup treatment -antimicrobial resistance Laboratory diagnosis • • Direct: – Microscopy: N.gonorrhoae in male – Culture:+antibiotic susceptibility – Antigen detection:rapid, less sensitive(Strep A/RSV/Rota../in urine: L. pneumophila) – NAAT: -real-time PCR(M.tuberculosis, Clostridium difficile, MRSA,VRE..) -multiplex-PCR, quantitation, resistance mutation detection Indirect: – spesific IGM: viral infections(measles,mumps,rubella.. – Quantitattive IgG: immunity: » Anti-Rubella IgG » Anti-HBs The success of the Microbiology laboratory • • • • • Quality of the specimen The way its sent The method used The interpretation: Do not hesitate to have contact with your microbiology laboratory! Reference: 7th ed 2013 Group A reportable disease • From every health care centers Group A reportable disease Group B reportable disease • Directly and promtly by phone • Internationally reported Group B reportable disease C Grubu Bildirimi Zorunlu Hastalıklar • From selected health care units Group C reportable disease Group D reportable disease • Positivity reported from the lab directly Group D reportable disease