* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download Chemical Disinfection
Cell-penetrating peptide wikipedia , lookup
Biochemistry wikipedia , lookup
Endomembrane system wikipedia , lookup
Protein adsorption wikipedia , lookup
List of types of proteins wikipedia , lookup
Gaseous signaling molecules wikipedia , lookup
Western blot wikipedia , lookup
Drug discovery wikipedia , lookup
Intrinsically disordered proteins wikipedia , lookup
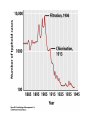
Chemical Disinfection 衛生署 疾病管制局 中區傳染病防治醫療網 王任賢 指揮官 Chemicals n n Use depends on spectrum of antimicrobial activity and compatibility with materials Also limited by dangers of chemicals themselves n Examples n n n n n n n Halogens Alcohols Alkylating agents Ethylene oxide Phenolics cetrimide (QAC) chlorhexidine (diguanide) The Ideal Disinfectant Ø Resistant to inactviation Ø Broadly active (killing pathogens) Ø Not poisonous (or otherwise harmful) Ø Penetrating (to pathogens) Ø Not damaging to non-living materials Ø Stable Ø Easy to work with Ø Otherwise not unpleasant Disinfectant殺菌效果之決定因素 Ø Disinfectant concentrations Ø Length (time) of administration Ø Temperature during administration (usual chemical reaction 2x increase in rate with each 10°C increase in temperature) Ø Microbe type (e.g., mycobacteria, spores, and certain viruses can be very resistant to disinfection—in general vegetative cells in log phase are easiest to kill) Ø Substrate effects (e.g., high organic content interferes with disinfection—stainless steel bench easier to disinfect than turd) Ø It is easier (and faster) to kill fewer microbes than many microbes Factors determining usefulness of chemical disinfection n Spectrum of antimicrobial activity n n is it the right agent for the job? Used at correct concentration n n n n concept of 'in use concentration’ diluted down from high concentration stored for <24 hours no topping up of old solutions Factors determining usefulness of chemical disinfection n Time of exposure n n n Correct pH? Inactivating materials n n You cannot disinfect an endoscope in 5 minutes glutaraldehyde! Pus, blood vomit, cork, soaps etc Is disinfectant sterile? n n Many cases of Gram-negatives living in disinfectants! Microbiological “in-use” testing Agent Mechanisms of Action Comments Moist Heat, boiling Denatures proteins Kills vegetative bacterial cells and viruses Endospores survive Moist Heat, Autoclaving Denatures proteins 121°C at 15 p.s.i. for 30 min kills everything Moist Heat, Pasteurization Denatures proteins Kills pathogens in food products Dry Heat, Flaming Incineration of contaminants Used for inoculating loop Dry Heat, Hot air oven Oxidation & Denatures proteins 170°C for 2 hours; Used for glassware & instrument sterilization Filtration Separation of bacteria from liquid (HEPA: from air) Used for heat sensitive liquids Cold, Lyophilization (also desiccation) Desiccation and low temperature Used for food & drug preservation; Does not necessarily kill so used for Long-term storage of bacterial cultures Cold, Refrigeration Decreased chemical reaction rate Bacteriostatic Osmotic Pressure, Addition of salt or sugar Plasmolysis of contaminants Used in food preservation (less effective against fungi) Radiation, UV DNA damage (thymine dimers) Limited penetration Radiation, X-rays DNA damage Used for sterilizing medical supplies Strong vis. Light Line-drying laundry Agent Mechanisms of Action Comments Surfactants Membrane Disruption; increased penetration Soaps; detergents Quats (cationic detergent) Denature proteins; Disrupts lipids Antiseptic - benzalconium chloride, Cepacol; Disinfectant Organic acids and bases High/low pH Mold and Fungi inhibitors; e.g., benzoate of soda Heavy Metals Denature protein Antiseptic & Disinfectant; Silver Nitrate Halogens Oxidizing agent Disrupts cell membrane Antiseptic - Iodine (Betadine) Disinfectant - Chlorine (Chlorox) Alcohols Denatures proteins; Disrupts lipids Antiseptic & Disinfectant Ethanol and isopropyl Phenolics Disrupts cell membrane Disinfectant, Irritating odor Aldehydes Denature proteins Gluteraldehyde - disinfectant (Cidex); Formaldehyde - disinfectant Ethylene Oxide Denaturing proteins Used in a closed chamber to sterilize Oxidizing agents Denature proteins Hydrogen peroxide – antiseptic; Hydrogen peroxide – disinfectan; Benzoyl peroxide – antiseptic Selection criteria (chemical antimicrobial agents) n n n n n n Antimicrobial efficacy Corrosivity Chemical hazard Environmental concerns Use solution pH Stability Biocidal spectrum Corrosivity Chemical hazard Environmental concerns Stability Surfactants Soap and Detergents n n n n Soaps are sodium or potassium salts of fatty acids, a natural product Detergents, instead, are artificial surfactants While soaps are always negatively charged, some detergents are negatively charged while others are positively charged One example of a positively charged detergent are quaternary ammonium compounds (a.k.a., quats) Chlorhexidine (a diguanide) n n n Used as general purpose antiseptic for skin and mucous membranes in many formulations, e.g. Hibiscrub, Hibisol, Savlon Advantages: relatively non-toxic and good against S. aureus Disadvantages: can support growth of e.g. P. aeruginosa Phenolics & QACs n n n Clear soluble phenolics (e.g. Hycolin) used as disinfectant on soiled surfaces, relatively inactive against spores and viruses Hexachlorophane used as surgical scrub Quaternary ammonium compounds, e.g. cetrimide usually only used in combination with other agents; good detergent properties. Phenols and Phenolics u Phenol (carbolic acid) was first used by Lister as a disinfectant. u u u u Phenolics are chemical derivatives of phenol u u u u Rarely used today because it is a skin irritant and has strong odor. Used in some throat sprays and lozenges (錠劑). Acts as local anesthetic. Cresols: Derived from coal tar (Lysol). Biphenols (pHisoHex): Effective against gram-positive staphylococci and streptococci. Used in nurseries. Excessive use in infants may cause neurological damage. Destroy plasma membranes and denature proteins. Advantages: Stable, persist for long times after applied, and remain active in the presence of organic compounds. Phenol, Carbolic Acid, & Phenolics n n n n n n Phenol (carbolic acid) and derivatives Affect plasma membrane, inactivates enzymes, and denature proteins Stable, persistant, and especially effective when dealing with disinfecting materials contaminated with organics… … but leave residual films, can irritate skin, don’t kill endospores, and are corrosive to rubber and plastics Some phenolics are mild enough for use as antiseptics while others are too harsh or otherwise dangerous to be employed on living tissue Hexachlorophene, Triclosan, Lysol, soap Quaternary Ammonium Compounds (Quats) u u u u u u u u Widely used surface active agents. Cationic (positively charge) detergents. Effective against gram positive bacteria, less effective against gram-negative bacteria. Also destroy fungi, amoebas, and enveloped viruses. Zephiran, Cepacol, also found in our lab spray bottles. Pseudomonas strains that are resistant and can grow in presence of Quats are a big concern in hospitals. Advantages: Strong antimicrobial action, colorless, odorless, tasteless, stable, and nontoxic. Diasadvantages: Form foam. Organic matter interferes with effectiveness. Neutralized by soaps and anionic detergents. Quats Ø Quats are cationic detergents that act by disrupting lipid bilayers Ø Quats are bactericidal, fungicidal, viricidal (enveloped), and amoebicidal Ø Quats are most effective against Gram-positive bacteria Ø Quats do not kill endospores, Mycobacteria spp., nor non-enveloped viruses Ø Quats are rapidly inactivated by organics including cotton and soap Ø Zephiran à Benzalkonium chloride Ø Cepacol àCetylpyridinium chloride Halogens n n Hypochlorites (household bleach) & chlorine Advantages n n active against viruses, spores, fungi Disadvantages n inactivated by organic matter, freshness & pH critical (go off if diluted), corrosive to metals n Practical Uses n n n n 0.1% hypochlorite used as general disinfectant Strong hypochlorite (0.25%) used in lab & on wounds Extra strong (1%) used on HBV blood spills Chlorine used to treat drinking water and control Legionella Halogens n n n n n n Two halogens are regularly employed as antimicrobials: Iodine and Chloride Iodine: commonly used as an antiseptic against all microbes, fungi, and viruses Iodine: It inhibits protein synthesis and oxidizes –SH groups of amino acids Chlorine: Used as a disinfectant (10% bleach) Chlorine: Hypochlorous acid (HOCl) is a product, formed in water, that is the active form of the disinfectant Chlorine: Applied in treatment of drinking water, swimming pool, and sewage Halogens n n Iodophors & iodine Advantages n n Disadvantages n n Some activity against viruses, spores, fungi inactivated by organic matter, can stain skin, irritant, expensive Practical Uses n n Pre-op skin disinfection Povidone iodine used as surgical scrub, as powder on ulcers Halogens Effective alone or in compounds A. Iodine: u Tincture of iodine (alcohol solution) was one of first antiseptics used. u Combines with amino acid tyrosine in proteins and denatures proteins. u Stains skin and clothes, somewhat irritating. u Iodophors: Compounds with iodine that are slow releasing, take several minutes to act. Used as skin antiseptic in surgery. Not effective against bacterial endospores. u Betadine u Isodine Halogens Effective alone or in compounds B. Chlorine: u When mixed in water forms hypochlorous acid: Cl2 + H2O ------> H+ + Cl- + HOCl Hypochlorous acid u u u u Used to disinfect drinking water, pools, and sewage. Chlorine is easily inactivated by organic materials. Sodium hypochlorite (NaOCl): Is active ingredient of bleach. Chloramines: Consist of chlorine and ammonia. Less effective as germicides Chlorination n n n n n n n n n n 1744 discovered in Sweden 1810 identified as an element 1835 first used to control odors 1890’s started to be used as a disinfectant 1896 earliest recorded use in experiments on water supplies 1897 used in England to sterilize water mains following typhoid outbreak 1902 first continuous use in water supplies in Belgium 1909 liquid chlorine (compressed gas) became commercially available Subsequent rapid spread in use of chlorine throughout the world WWI: Chlorine gas used as chemical warfare ag Chlorination and Typhoid fever Chlorination n n n n n n n Hypochlorite may either be added directly (i.e., in the form of bleach) or created within water by bubbling chlorine gas through the water Chlorine gas - preferred for medium to large disinfection systems Sodium Hypochlorite (liquid) - typically used for small disinfection systems and large swimming pools Calcium Hypochlorite (powder, tablet) - typically used for private swimming pools For water purification, do not use scented bleach Bromine sometimes used as a less-smelly alternative Hypochlorite is less effective in the presence of significant organic compounds Chlorination n n “What is known as modern chemical warfare began during World War I. The first chemical agent to be used was large amounts of chlorine gas, about one hundred sixty tons, which was released from 6,000 pressurized cylinders into the wind by the Germans against the Allies. The chlorine floated in a huge clouds toward the Allies until it reached the Allied lines causing men to die from the effects of the chlorine gas. Because of the large amounts of gas released the chlorine caused large amounts of yellowish fluid to form in the lungs of its victim, also causing eye, nose, and throat burning before causing death by choking.” http://www.geocities.com/CapeCanaveral/Lab/4239/chemweapo ns/history.html Iodine Iodophores Alcohols n n Isopropanol & ethanol Advantages n n Disadvantages n n n n kill vegetative bacteria on clean surfaces in 30 seconds inactive against spores, fungi Inflammable Need to be at correct % with water (65-80%) Practical uses n n n Skin antisepsis before venepuncture Hand rubs Disinfection of e.g. trolley tops Alcohols u u u u u Kill bacteria, fungi, but not endospores or naked viruses. Act by denaturing proteins and disrupting cell membranes. Evaporate, leaving no residue. Used to mechanically wipe microbes off skin before injections or blood drawing. Not good for open wounds, because cause proteins to coagulate. u u Ethanol: Drinking alcohol. Optimum concentration is 70% Isopropanol: Rubbing alcohol. Better disinfectant than ethanol. Also cheaper and less volatile. Alcohols n n n n n n n Aqueous ethanol (60-95%) and isopropanol are used as disinfectants Effectively kill bacteria and fungi but not endospores nor nonenveloped viruses Fast acting, no residue (evaporate away), no staining But not very penetrating and no residual activity (once gone gone) Exert their action by denaturing proteins and dissolving lipids In tinctures, they enhance the effectiveness of other antimicrobial chemicals Flammable; also may damage rubber, plastic, etc. Tinctures A tincture is a nonvolatile substance (medicine) presented as an alcohol solution, e.g., (for fun with numerous [sic])… Tincture Ø “Formulae: Fresh juice of Organic Habanero peppers, New Mexico Jalapeno, African Bird peppers and Hatch Chili peppers. Ø Dosage: Five to thirty drops, three times daily. CAUTION ~ EXTREMELY HOT!! Ø Therapeutic Action: Cayenne is the greatest herbal aid to circulation and should be used on a regular basis. The extract is very concentrated and gets into the bloodstream quickly and makes it a perfect first aid remedy for heart attacks, stroke, fainting, shock, dizziness, hemorrhage, internal and external bleeding. Use a few drops to 10 droppers full. It has saved many lives. Tincture II Tincture II n n n “Formulae: Fresh Garlic Juice, Goldenseal root, Usnea lichen, Myrrh gum, Pine resin, Echinacea root juice, Tea Tree oil, Kelp, Black Walnut inner hulls, Oak galls and Cayenne pepper in 80% grain alcohol. Dosage: Generally for external use but can be used in the oral cavity. Soak a cotton swab in the tincture and scrub into the infected area, let air dry. It has a burning sensation. If the wound is tender, just flush it with multiple droppers full of the tincture but no need to scrub it in. Therapeutic Action: There has never been an infected occur when this formula has been used. It's excellent for treating any cut or wound and it is anti-bacterial, anti-viral and anti-fungal. The tree resins in this formula leave an invisible protective, anti-bacterial coating over the wound. This formula was use on a man in England who had the top of his knee torn off in an automobile accident. In 24 hours it literally glued his knee back together. A nurse from Ireland on the scene said in all the years in the hospital, she had never seen such a severe wound close right up and heal, and with no infections.” Heavy Metals n n n n Ag, Cu, Hg, Ni, Zn, Ag(NO3)2, CuSO4, ZnCl2, HgCl2 These metals (and metal ions) react with sulfhydral (–SH) groups of proteins, denaturing proteins Silver nitrate is used to treat Ophthalmia neonatorum in newborns as caused by Neisseria gonorrhoeae Oligodynamic action: the ability of very small amounts of heavy metals (especially silver and copper) to exert antimicrobial activity Heavy Metals Include copper, selenium, mercury, silver, and zinc. u Oligodynamic action: Very tiny amounts are effective. A. Silver: u 1% silver nitrate used to protect infants against gonorrheal eye infections until recently. B. Mercury u Organic mercury compounds like merthiolate and mercurochrome are used to disinfect skin wounds. C. Copper u Copper sulfate is used to kill algae in pools and fish tanks. u Heavy Metals D. Selenium (硒) u Kills fungi and their spores. Used for fungal infections. u Also used in dandruff (去頭皮屑) shampoos. E. Zinc u Zinc chloride is used in mouthwashes. u Zinc oxide is used as antifungal agent in paints. Alkylating agents n n Glutaraldehyde and Formaldehyde Advantages n n Disdvantages n n n n n Good activity against spores, virues, fungi Glutaraldehyde only moderately active against TB Need long exposure time for full effect (3 hours) freshness & pH critical TOXIC! Practical uses n n n Disinfection of endoscopes Blood spills Fumigation Aldehydes Include some of the most effective antimicrobials. u Inactivate proteins by forming covalent crosslinks with several functional groups. A. Formaldehyde gas: u Excellent disinfectant. u Commonly used as formalin, a 37% aqueous solution. u Formalin was used extensively to preserve biological specimens and inactivate viruses and bacteria in vaccines. u Irritates mucous membranes, strong odor. u Also used in mortuaries for embalming. u Aldehydes B. Glutaraldehyde: u Less irritating and more effective than formaldehyde. u One of the few chemical disinfectants that is a sterilizing agent. u A 2% solution of glutaraldehyde (Cidex) is: u u u u Bactericidal, tuberculocidal, and viricidal in 10 minutes. Sporicidal in 3 to 10 hours. Commonly used to disinfect hospital instruments. Also used in mortuaries for embalming. Glutaraldehyde Glutaraldehyde is capable of effectiving sterilization—at room temperature, even against endospores, and even in the presence of organics, but achieving sterilization requries many hours of exposure… and it is nasty stuff to work with! Gaseous Chemosterilizers n n n n n Propylene oxide (C3H6O) Chlorine gas (Cl2) Chlorine dioxide (ClO2) Ozone (O3) Ethylene oxide (C2H4O)… Gaseous Chemosterilizers n n n n n n to sterilize heat- or moisture-sensitive items for items damaged by heat or moisture is not corrosive, not damaging to delicate instruments, microscopes, disposable plastic instruments and materials permeates porous materials dissipates rapidly from material but is costly, toxic, carcinogenic, explosive, and relatively lengthy process Gaseous Sterilizers Chemicals that sterilize in a chamber similar to an autoclave. u Denature proteins, by replacing functional groups with alkyl groups. A. Ethylene Oxide: u Kills all microbes and endospores, but requires exposure of 4 to 18 hours. u Toxic and explosive in pure form. u Highly penetrating. u Most hospitals have ethylene oxide chambers to sterilize mattresses and large equipment. u Ethylene oxide n n n n Highly toxic flammable gas, kills spores! Used for bulky items such as heart lung machines Can be used on glutaraldehyde-labile endoscopes Use limited by safety issues Oxidizing Agents n n n n n n HOOH, hydrogen peroxide, is most common HOOH is not a terribly effective disinfectant or anticeptic This is because bacteria and body tissues contain enzymes (catalase) that inactivate hydrogen peroxide However, the oxygen released upon inactivation can help oxygenate deep wounds and thus kill strictanaerobe contaminants, e.g., Clostridium tetani Ozone and peracetic acid are also oxidizing antimicrobial agents They exert their effect by oxidizing cell macromolecules (e.g., proteins, DNA, etc.) Peroxygens (Oxidizing Agents) Oxidize cellular components of treated microbes. u Disrupt membranes and proteins. A. Ozone: u Used along with chlorine to disinfect water. u Helps neutralize unpleasant tastes and odors. u More effective killing agent than chlorine, but less stable and more expensive. u Highly reactive form of oxygen. u Made by exposing oxygen to electricity or UV light. u Peroxygens (Oxidizing Agents) B. Hydrogen Peroxide: u Used as an antiseptic. u Not good for open wounds because quickly broken down by catalase present in human cells. u Effective in disinfection of inanimate objects. u Sporicidal at higher temperatures. u Used by food industry and to disinfect contact lenses. C. Benzoyl Peroxide: u Used in acne medications. Peroxygens (Oxidizing Agents) D. Peracetic Acid: u One of the most effective liquid sporicides available. u Sterilant : u u u Kills bacteria and fungi in less than 5 minutes. Kills endospores and viruses within 30 minutes. Used widely in disinfection of food and medical instruments because it does not leave toxic residues. Efficiency of Different Chemical Antimicrobial Agents Chemical Antimicrobials * Type of Disinfectant: H = High level; I = Intermediate level; L = Low level The Problem of CJD and TSEs n n n Creutzfeld-Jakob syndrome and other transmissible spongiform encephalopathies caused by highly resistant proteinaceous particles, prions can survive 3 years of environmental exposure and are unusually resistant to conventional decontamination methods Iatrogenic CJD documented in three circumstances n n n use of contaminated medical equipment (2 cases) use of extracted pituitary hormones (> 130 cases) implantation of contaminated grafts from humans (cornea, 3 cases; dura mater, > 110 cases) 懇請賜教