* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download How many valence electrons does gold have? For the d
Oxidation state wikipedia , lookup
Bond valence method wikipedia , lookup
Hydroformylation wikipedia , lookup
Jahn–Teller effect wikipedia , lookup
Cluster chemistry wikipedia , lookup
Metal carbonyl wikipedia , lookup
Evolution of metal ions in biological systems wikipedia , lookup
Spin crossover wikipedia , lookup
Metalloprotein wikipedia , lookup
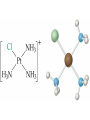
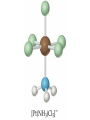
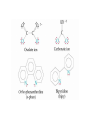
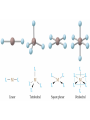
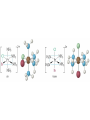
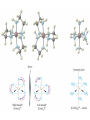
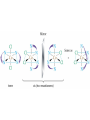
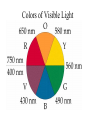
Transition Metals and Coordination Compounds Transition Metals • The transition metals are the d-block elements. • The Inner Transitions metals are the lanthanides and actinides, or the f-block. • Many transition metals form highly colored compounds. • Many are critical for our health! d-block & Valence Electrons • How many valence electrons does iron have? • How many valence electrons does gold have? • For the d-block, there are (n-1)d + ns valence electrons. • Why do we count the d electrons??? d-block & Valence Electrons • How about ions? • How many valence electrons does Cu have and how many does Cu+1 have? • What are their electron configurations? Oxidation States • Many transition metals are found in more than 1 oxidation states, even Ag and Zn! • Here are some common states: Oxidation States • We can assign oxidation states using the rules you already learned to love! • Determine the oxidation states for the transition metals in the following complexes: Coordination Compounds • The preceding complexes are examples of coordination compounds or coordination complexes. • Note that they may be neutral or charged. • What do you notice? What do you think is the central atom? Coordination Compounds • In coordination compounds, the central atom is a metal atom or ion, and it is covalently attached to 2 or more molecules or ions. • The molecules or ions covalently attached to the transition metal are called ligands. Coordination Compounds • In a ligand, the atom(s) which are directly attached to the metal are called the ligand donor atoms or simply the donor atoms. • Look at the following and determine what the ligands are, and what the donor atoms are. Complex Ions • If the coordination compound has a charge, it is a complex ion. • We write it in brackets with the charge outside. • But if it’s an ion, what can it do? • Complex ions can form ionic salts. • How do we write the formulas for these complex salts? Coordination Numbers • We also talk about coordination numbers for coordination compounds. • The coordination number for a transition metal is the number of ligand donor atoms directly attached to it. Coordination Numbers • Common coordination numbers are 2, 4, or 6, but they may be anything from 2 to 8 (including odd). Coordination Numbers • What is the coordination number for the following: Common Ligands • Ligands may actually form more than 1 bond or attachment to the transition metal. • Ligands which form only 1 bond are called monodentate ligands. • Ligands which form more than 1 bond are called polydentate ligands. • Bidentate ligands are fairly common. Common Ligands • Let’s look at some polydentate ligands and how they bond or attach. Common Ligands • You do need to know the most common ligands so you can name transition compounds. • The following table has all that you need to know except the pyridine ligand which is abbreviated py. Structure • Before we switch to nomenclature, what about the structures? • As the most common coordination numbers are 2, 4, and 6, there are 4 common geometric shapes: – – – – linear tetrahedral square planar octahedral Structure • What’s the transition metal hybridization associated with these shapes? Nomenclature • There are some rules to name the simpler coordination compounds. 1. If the complex is a salt, name the cation portion first, then name the anion. Nomenclature 2. When naming a complex ion or a neutral complex, name the ligands using the appropriate prefix (di, tri, tetra, etc), then name the metal. If a ligand is an anion, it name ends in -o (like chloro or cyano). Specify the metal oxidation # with a Roman numeral inside ( ). If there are more than 1 different ligands, name them alphabetically, ignoring the prefixes. Nomenclature 3. If the ligand atom itself contains a prefix (for you this is ethylenediamine), then put the name inside ( ), and use one of the following prefixes to specify the number of these ligands: 2 is bis, 3 is tris, 4 is tetrakis. 4. If the complex ion is an anion, give the metal an -ate ending. Isomers • Isomers are compounds with the same molecular formula but have the atoms arranged differently. There are several types of isomers: • – – – Conformational or Structural Geometric or Diasteorisomers Enantiomers Transition Metals and Color • Why are so many transition metal complexes colored? • Usually only metals with d0 or d10 form colorless compounds (Zn, Ag). • Colors in metal complexes (or any compound) is due to their absorption spectrum. Transition Metals and Color • When a metal complex absorbs light, an electron undergoes an electronic transition from a ground state to an excited state. • Remember: ∆E = hc/ OR = hc/E • We see the color which is NOT absorbed, but which was reflected or transmitted. • We see the complementary color of what was absorbed. Colors of Visible Light Crystal Field Theory • Why do metal complexes absorb light in the Vis light spectrum? • Crystal Field Theory tries to explain this. • When a ligand approaches a free metal atom or ion in order to form a bond, e-e repulsions occur between the metal’s d-electrons and the ligands electrons. • This causes the metal’s 5 degenerate dorbitals to increase in energy AND to split. • So they are no longer degenerate. d-Orbital Splitting by Ligands Octahedral Complexes and Orbitals Crystal Field Theory • Different ligands cause more of an energy split in the d-orbitals. • Ligands which cause the d-orbitals to split more with a higher E are called strong-field ligands. • Ligands which cause the d-orbitals to split less with a lower E are called weak-field ligands. • Ligand Series from Weak to Strong: I-<Br-<Cl-<F-<H2O<NH3<en<CN- Octahedral Complexes: Weak Field / Strong Field Ligands Crystal Field Theory • Ligand splitting of a metal’s d-orbitals also explains why some complexes are highly paramagnetic and others are diamagnetic or weakly paramagnetic. • Highly Paramagnetic: These are weak field complexes with a low E, so the d-e are easily promoted. The result is a complex with many unpaired electrons, or a high-spin complex. Tetrahedral & Square Planar Complexes