* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download bright field microscopy
Vibrational analysis with scanning probe microscopy wikipedia , lookup
Optical tweezers wikipedia , lookup
Upconverting nanoparticles wikipedia , lookup
Night vision device wikipedia , lookup
Astronomical spectroscopy wikipedia , lookup
Thomas Young (scientist) wikipedia , lookup
Retroreflector wikipedia , lookup
Anti-reflective coating wikipedia , lookup
Atmospheric optics wikipedia , lookup
Fluorescence correlation spectroscopy wikipedia , lookup
Ellipsometry wikipedia , lookup
Surface plasmon resonance microscopy wikipedia , lookup
Photon scanning microscopy wikipedia , lookup
Preclinical imaging wikipedia , lookup
Phase-contrast X-ray imaging wikipedia , lookup
Dispersion staining wikipedia , lookup
Gaseous detection device wikipedia , lookup
Ultrafast laser spectroscopy wikipedia , lookup
Chemical imaging wikipedia , lookup
Ultraviolet–visible spectroscopy wikipedia , lookup
Optical coherence tomography wikipedia , lookup
Magnetic circular dichroism wikipedia , lookup
Scanning electron microscope wikipedia , lookup
Nonlinear optics wikipedia , lookup
Harold Hopkins (physicist) wikipedia , lookup
Confocal microscopy wikipedia , lookup
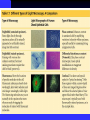
TYPES OF MICROSCOPES
1.
2.
3.
Mikroskop Cahaya (Visible Light
Microscope)
Fluorescence Microscope (Mikroskop
Fluoresen)
Electron Microscope
Visible Light Microscope
1.
2.
3.
4.
5.
Brightfield Microscopy
Darkfield Microscopy
DIC (Difference Interference Contrast)
Phase Contrast Microscopy
Polarisasi Microscopy
Fluorescence Microscope
1.
2.
Epi-fluorescence
Confocal Laser
Scanning
Microscope
BRIGHT FIELD MICROSCOPY
• Most commonly used microscopy imaging technique is bright field
microscopy, where light is either passed through or reflected off a specimen
• Biologists and histologists have used counter staining for over one hundred
years; and this helps to differentiate the various tissues and organelles that
can be found in a variety of subjects that would otherwise be rendered
invisible
Drawbacks:
The cells are usually killed and therefore cannot be studied whilst moving
around their natural habitat
When to use bright field microscopy
• Viewing stained or naturally pigmented specimens such as stained prepared
slides of tissue sections
• Used when there is enough contrast in the subject matter or artificial staining
techniques are employed .
BRIGHT FIELD
Main uses:
• Viewing stained specimens
• Pathological exams
• Blood tests
• Water inspections
• Liquid crystal board inspections
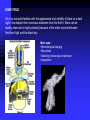
Brightfield Requirements
Condenser
Objectives
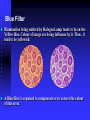
Blue Filter
Illumination being emitted by Halogen Lamp tends to be on the
Yellow Hue. Colour of image are being influence by it. Thus, it
tend to be yellowish.
A Blue filter is required to compensate or to correct the colour
of this error.
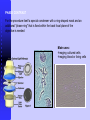
DARK FIELD MICROSCOPY:
• A special condenser lens is used to illuminate the specimen diagonally, then observe
light scattering off it.
• The field of view is darker than bright field microscopy because illumination light does
not enter the objective lens.
• Oblique illumination is used to increase the visibility of specimens
• Useful in revealing very fine detail especially bacteria
Drawbacks:
• Dark field is only black and white and is missing the information from the shading ability
of phase contrast
• For serious dark field work, one needs to use a dedicated darkfield condenser.
DARK FIELD
All of us are quite familiar with the appearance and visibility of stars on a dark
night, this despite their enormous distances from the Earth. Stars can be
readily observed at night primarily because of the stark contrast between
their faint light and the black sky.
Main uses:
•Microbiological imaging
•Blood tests
•Detecting microscopic scratches or
irregularities
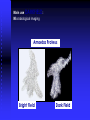
Main use DARKFIELD:
Microbiological imaging
Amoeba Proteus
Bright Field
Dark Field
Darkfield Requirements
Darkfield Condenser
PHASE CONTRAST MICROSCOPY:
• Optical phenomena of diffraction and interference are used to add light/dark
contrast to a transparent specimen for imaging.
• There is no need to stain the specimen as in brightfield microscopy, so live
specimens can be used.
• Enhances contrasts of transparent and colorless objects by influencing the
optical path of light
Drawbacks:
A disadvantage of this method is the appearance of light halos around some
objects ("halo-effect").
When to use Phase Contrast microscopy
• Phase contrast is preferable to bright field microscopy when high
magnifications (400x, 1000x) are needed and the specimen is colorless or the
details so fine that color does not show up well.
• Cilia and flagella, for example, are nearly invisible in bright field but show up
in sharp contrast in phase contrast
PHASE CONTRAST
For the procedure itself a special condenser with a ring-shaped mask and an
additional "phase-ring" that is fixed within the back focal plane of the
objective is needed
Main uses:
•Imaging cultured cells
•Imaging blood or living cells
Phase Contrast Requirements
Phase
Contrast
Turret
Condenser
Green
Interference
Filter
Centering
Device
Phase
Contrast
Objective
DIFFERENTIAL INTERFERENCE CONTRAST MICROSCOPY (D.I.C)
• Transforms minute differences in refraction indexes of light passing through an
unstained specimen, or optical path differences from the specimen surface shape, into a
monochromatic shadow-cast image enabling observation.
• 3D-pseudo effect that it gives and also, unlike phase contrast there are no halos around
the subject
Drawbacks:
• DIC utilizes optical path differences within the specimen (i.e.: product of refractive index
and geometric path length) to generate contrast the three-dimensional appearance may
not represent reality
• Birefringent specimens such as those found in crystals may not be suitable because of
their effect upon polarized light. Similarly, specimen carriers, such as culture vessels,
Petri dishes, etc., made of plastic may not be suitable
When to use DIC?
As with phase contrast microscopy, DIC microscopy may be used with living specimens.
However, it is better suited to thicker specimens.
DIC
The DIC set-up consists of:
A Polarizer,a Wollaston prism the Object, Wave train, Objective, Wollaston
prism, Analyzer, and Eyepiece.
Main uses:
•Imaging fibrous structure of nerve
•Imaging mitotic spindles
•Imaging cellular nucleic structures or
other thick unstained specimens
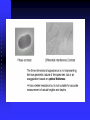
Example of DIC image
The stereoscopic effect unique to
DIC is observed in this example.
A
B
C
Retardation
A>B>C
Nematode
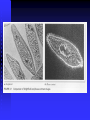
Example of DIC image
Contrast has directivity.
Volvox
Example of DIC image
The specimen was observed
using both fluorescence and DIC
microscopy.
Salivary gland of drosophila
DIC Requirements
Universal DIC
Condenser
Polarizer
DIC Elements
Analyzer
DIC Slider
DIC vs Phase Contrast
DIC
Phase Contrast
DIC
PHASE CONTRAST
Higher revolving power
Lower revolving power
(40X: 0.95 / 60X: 1.30-1.42)
(40X: 0.75 / 60X: 1.25)
Thicker specimen
Only for thinner specimen
Good with Fluorescence
Not good with Fluorescence
Glass only
Plastic and Glass
POLARIZING MICROSCOPY
• This technique uses the phenomenon of polarization to add contrast and color to
specimen images.
• Designed to observe and photograph specimens that are visible primarily due to their
optically anisotropic character.
• When this beam passes through certain specimens the plane of the waves is "rotated".
In some cases the extent of rotation varies with wave length, or "colour" (birefringence)
• Second filter, referred to as the analyser, prior to viewing
• When a birefringent specimen is viewed under these conditions, the rotated light can
pass through the analyser
Drawbacks:
Proper alignment of the various optical and mechanical components is a critical step that
must be conducted prior to undertaking quantitative analysis between crossed polarizers
alone, or in combination with retardation plates and compensators
When to use Polarizing microscopy
Polarized light is a contrast-enhancing technique that improves the quality of the image
obtained with birefringent materials when compared to other techniques
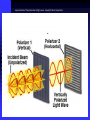
The basic configuration of polarized optical microscope. Copyright: Nikon Corporation.
A schematic
representation of the polarization of light waves. Copyright: Nikon Corporation.
A schematic representation of a Nicol polarzing prism. Copyright: Nikon Corporation.
Microscope must be equipped with both a polarizer, positioned in the light
path somewhere before the specimen, and an analyzer (a second polarizer),
placed in the optical pathway between the objective rear aperture and the
observation tubes or camera port.
POLARIZING
Incident light is
polarized, passes
through the sample and
crossed polar analyzer
to an image of a brightly
colored (interference
colored) image of the
pigment crystallite.
Main uses:
•Analysis of optical properties of rocks, ores
•Polarization analysis of fine structures within
living organisms and cytoskeletons
•Gout testing
Simple Polarizing
Requirements
Polarizer
Analyzer
Polarizing Requirements
For Measurement of
various retardation
For identification of orthoscopic &
conoscopic materials
Relief Contrast
A contrast technique made possible by using
a special aperture located in the objective
and condenser.
ICSI procedure
Relief Contrast Requirements
• Relief Contrast Condenser
RC Modulator for 10x, 20x, 40x,
FLUORESCENCE MICROSCOPY
•Specimen is excited with a specific wavelength of light, then fluorescent emissions are
observed.
Drawbacks
•Photo bleaching can significantly cause measurement error
When to use Fluorescence microscopy
•Used to study specimens, which can be made to fluoresce.
•Certain material emits energy detectable as visible light when irradiated with the light of
a specific wavelength. The sample can either be fluorescing in its natural form like
chlorophyll and some minerals, or treated with fluorescing chemicals.
FLUORESCENCE
WHAT IS FLUORESCENCE?
What is Fluorescence?
Light of a short wavelength generates
light of a longer wavelength.
Jablonski diagram
Illustrating the processes involved in
the creation of an excited electronic
singlet state by
optical absorption and subsequent
emission of fluorescence.
Upon absorbing the excitation light,
usually of short wavelengths, electrons
may be raised to a higher energy and
vibrational excited state
excited electrons lose some energy & return to
the lowest excited singlet state with simultaneous
emission of fluorescent light
Fluorescent Organic Dyes
Inorganic Fluorophors
High quantum efficiency.
Poorer quantum efficiency.
More limited selection.
Many colors to choose from.
Conjugated to anti-bodies
and proteins.
Highly stable.
Less sensitive to changes in
temperature.
Many sensitive to pH,
When in host, not sensitive to
temperature,solvents, etc.
pH, moisture,or solvents.
Photo-bleaching a problem.
Makes great standards!
Even poor thermal stability
once in solution..
FLUORESCENCE
•Sample you want to study is itself the light source.
Main uses:
•Imaging and quantification
•Assaying antigens in antigen/antibody reactions
•Imaging and quantification of intracellular DNA
•Analysis of chromosomal abnormalities
Principle of Fluorescence
1. Energy is absorbed by the atom which becomes excited.
2. The electron jumps to a higher energy level.
3. Soon, the electron drops back to the ground state, emitting
a photon (or a packet of light) - the atom is fluorescing
Properties of Fluorochrome
Fluorochrome
Excitation (nm)
Emission (nm)
Color
DAPI
365
420
Blue
Fluorescein
495
525
Green
Hoechts 33258
360
470
Blue
R-phycocyanin
555, 618
634
Red
B-phycoerythrin
545, 565
575
Orange, red
R-phycoerythrin
480, 545, 565
578
Orange, red
Rhodamine
552
570
Red
Texas red
596
620
Red
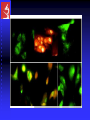
Aktin-mitokondria
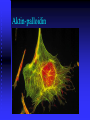
Aktin-palloidin
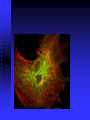
Endotel-mitokondria
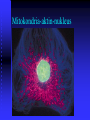
Mitokondria-aktin-nukleus
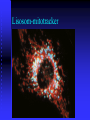
Lisosom-mitotracker
IMAGE COMPONENTS