* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download Responsible Oversight Strategies for Genome - NAS
Genomic library wikipedia , lookup
Human–animal hybrid wikipedia , lookup
Designer baby wikipedia , lookup
Genetically modified organism containment and escape wikipedia , lookup
Genome evolution wikipedia , lookup
Microevolution wikipedia , lookup
Genetically modified food wikipedia , lookup
Genetic engineering wikipedia , lookup
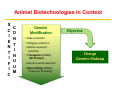
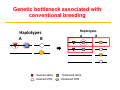
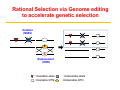
Responsible Oversight Strategies for Genome Editing Technology in Agricultural Animals in the United States Bhanu Telugu DVM.PHD Susan Harper, DVM (USDA-ARS) Diane Wray-Cahen PHD (USDA-FAS) Overview • Introduction and Rationale for performing genome editing • Discuss current Regulatory landscape • Provide a case study Animal Biotechnologies in Context S C I E N T I F I C C O N T I N U U M Genetic Modification Objective ▪ Mass selection ▪ Pedigree selection ▪ Marker‐assisted selection ▪ Transgenics (1980s) (GE Animals) ▪ Genome‐wide selection ▪ Gene Editing (2000s) “Precision Breeding” Change Genetic Makeup Rationale for genome editing in livestock for agricultural applications Current strategySelective breeding is not pragmatic Genetic bottleneck associated with conventional breeding Haplotypes A B : Desired allele; : Desired QTN; Haplotypes A B :Undesired allele :Undesired QTN Rational Selection via Genome editing to accelerate genetic selection Deletion (NHEJ) T G Replacement (HDR) : Desirable allele; : Desirable QTN; :Undesirable allele :Undesirable QTN Rationale for Genome editing over Conventional Breeding Genome editing: 1. Gene deletion (knockout) 2. Gene modification 3. insertion (knockin) Rationale: – Overcome otherwise low heritability – Separate “linked” genes – Increase precision and efficiency of introducing desirable traits (conventional breeding is random) – Introduction of traits not available via conventional breeding Rationale for Genome editing Gene deletion- knockouts CRISPR/cas9 + sgRNA mRNA All piglets edited!! Genome Editing Potential Benefits • Facilitates precise modifications • Faster, more reliable, and cheaper than conventional breeding (long generation interval) to modify base pairs of genes Potential Risks • Off-target edits • Reducing genetic diversity in genome edited herds • Potential spread of edited genetic material into non-target or closely related populations (biocontainment)- issue with some than others Additional Legal and Ethical Concerns • Perceived novel environmental risks (Potential benefit‐ Enviropigs; Potential risks?) • Misuse of technology for entertainment purposes and potentially nefarious reasons (Eg.,micropigs as pets in China; HOW IS DIFFERENT FROM DESIGNER DOG BREEDING) • Application of technology developed in animals to human germline editing (NOTE: not somatic editing) How to Regulate Genome Edited Animals ? Mid‐1980’s expression of need for establishment of regulatory oversight in light of the transgenic animals that have been generated Regulation of Biotechnology in the United States Regulation Under the Coordinated Framework USDA Safe for the animal, agriculture and the environment FDA Safe for use in food and feed EPA Safe for environment Coordinated Framework – General Principles Federal Role in the Safe Use of Biotechnology • USDA does not regulate GE animals for Food production. It is the role of FDA [FDA has emerged as a de facto enforcer]. • The risks of genetically engineered (GE) organisms are not fundamentally different from the risks posed by non-GE organisms with similar traits. • The existing laws provide adequate authority. • Regulation should be science-based and conducted on a caseby-case basis (Need to be revisited for Editing animals). How are GE animals regulated in United States? Transgenic animals that have recombinant DNA (trigger) have clear guidelines • In 2009, the Food and Drug Administration (FDA) issued Guidance: Regulation of Genetically Engineered Animals Containing Heritable Recombinant DNA Constructs • The FDA regulates the recombinant DNA construct as a “new animal drug”, “an article intended to alter the structure or function” of the animal (trigger) • New animal drug approval is based on a showing that the product is “safe” (for animals, humans, and the environment) and “effective” for the intended use APHIS FDA FDA Commercialization of Animal Biotechnology Transgenic models (mice, rats, zebrafish) GloFish (2003) [Enforcement Discretion] Atryn Goat (2009, USA) Project on hold X Oxitec mosquito (2014, Brazil) Enviropig (Canada) Finally approved Commercialization ? AquAdvantage Salmon (USA) How to regulate edited animals that do not have recombinant DNA (trigger), or that are similar to naturally occurring sequences? Existing Regulatory Frameworks • Many provisions intended for the regulation of conventional transgenic animals do not necessarily directly apply to animals produced through genome editing technologies. • Regulations are already in place to protect the welfare and safety of animals, workers, the environment, and the public. • Local oversight of genetic research involving animals includes: • Institutional Animal Care and Use Committee (IACUC) • Institutional Biosafety Committee (IBC) Institutional Animal Care and Use Committee (IACUC) • Required by: • Animal Welfare Act Regulations (AWAR) when regulated species are used for research, testing, or teaching • PHS Policy when PHS funding is received • NRC Guide for the Care and Use of Laboratory Animals and FASS Ag Guide • Establishes local oversight of the welfare and safety of animals used for research, teaching, and/or testing • Incorporates a harm‐benefit analysis to ensure the use of animals is justified • USDA‐APHIS Animal Care Policy No. 10 requires facilities that produce cloned animals for regulated purposes to be licensed, and those that produce novel genetically engineered animals to be registered as research facilities. • AWAR exempts agricultural animals used in food and fiber research and federal research programs. Institutional Biosafety Committee (IBC) • NIH Guidelines requirement for institutions that receive NIH funding • Establishes local oversight for research involving recombinant or synthetic nucleic acid molecules • NIH Guidelines requirements for transgenic animals include: • Identification and disposal guidance for animals larger than traditional laboratory animals (e.g., cattle, swine, sheep, goats, horses, and poultry) • Permanent marking of genetically engineered offspring within 72 hours of birth, if their size permits • Distinct and biochemically assayable DNA sequences that permit transgenics to be identified from non‐transgenic animals • Containment and confinement practices Miscellaneous Regulations • Clean Water Act (33 USC 1251) with respect to contaminated runoff from animal production facilities • Occupational Safety and Health Administration (OSHA) workplace requirements • CDC‐NIH “Biosafety in Microbiological and Biomedical Laboratories” (BMBL) laboratory safety requirements • State and Local Regulations related to environmental protection and worker safety • Relevant International Standards Regulatory framework adapted at ARS, USDA for an editing project • Goal is to produce pigs that do not serve as a reservoir for influenza. • Foundation pigs will be used to produce offspring. • Influenza challenge studies will be performed in offspring, requiring ABSL‐3 containment. • Animals will be monitored for adverse genotypic and phenotypic stability, as well as unintended or adverse characteristics. Regulatory framework adapted at ARS, USDA for an editing project • Project has been reviewed by IACUC and IBC at the University and two ARS locations. • Review process emphasized potential animal welfare implications, biosecurity concerns, laboratory safety, potential occupational health issues, and containment/confinement practices. • Agreements were established to clarify specific institutional responsibilities, animal ownership, and oversight roles. • No gaps perceived in current local and government oversight processes. Acknowledgements Funding Sources For More Information “Unified” website for U.S. Regulatory Agencies under the Coordinated Framework: http://usbiotechreg.epa.gov/usbiotechreg/ USDA-APHIS-BRS: http://www.aphis.usda.gov/biotechnology/index.shtml EPA: http://www.epa.gov/pesticides/biopesticides/pips/index.htm FDA-CFSAN: http://www.fda.gov/Food/Biotechnology/default.htm