* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download APPROACH TO CYANOTIC CONGENITAL HEART DISEASE IN
History of invasive and interventional cardiology wikipedia , lookup
Management of acute coronary syndrome wikipedia , lookup
Cardiac contractility modulation wikipedia , lookup
Electrocardiography wikipedia , lookup
Coronary artery disease wikipedia , lookup
Heart failure wikipedia , lookup
Mitral insufficiency wikipedia , lookup
Myocardial infarction wikipedia , lookup
Cardiothoracic surgery wikipedia , lookup
Hypertrophic cardiomyopathy wikipedia , lookup
Lutembacher's syndrome wikipedia , lookup
Cardiac surgery wikipedia , lookup
Quantium Medical Cardiac Output wikipedia , lookup
Congenital heart defect wikipedia , lookup
Arrhythmogenic right ventricular dysplasia wikipedia , lookup
Atrial septal defect wikipedia , lookup
Dextro-Transposition of the great arteries wikipedia , lookup
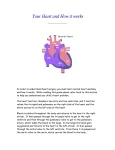
APPROACH TO CYANOTIC CONGENITAL HEART DISEASE IN THE NEWBORN By Christopher Cheung, MD 2013 Reviewed by: Santokh Dhillon, MD, MBBS INTRODUCTION TO CYANOSIS Cyanosis is a bluish or purple discoloration of the skin and mucous membranes associated with poor oxygenation. It is noticeable when >5 g/dL of deoxygenated hemoglobin is present and usually assessed by pulse oximetry. It is very difficult to detect unless the arterial saturation is ≤ 85% and best seen in tongue and oral mucosa. Acrocyanosis (cyanosis only in peripheral parts), commonly seen with cold environments or after bathing, is always a normal finding and is not a true cyanosis. Long standing cyanosis results in digital clubbing. Causes of Cyanosis: Cardiac and Non-Cardiac Non-Cardiac Causes of Neonatal Cyanosis Pulmonary Hyaline membrane disease Meconium aspiration/Pneumonia Pneumothorax/Pleural effusion Persistent pulmonary hypertension of the newborn Choanal atresia or stenosis Diaphragmatic hernia Neurologic CNS dysfunction Respiratory neuromuscular dysfunction Heavy maternal sedation Perinatal asphyxia Intrauterine fetal distress Please note that an in-depth approach to neonatal cyanosis is available on LearnPediatrics.com Cardiac causes of cyanosis can be divided into ductal-dependent and ductal-independent lesions. Ductal-dependent lesions require the ductus arteriosus for adequate pulmonary circulation and include: Tetralogy of Fallot, tricuspid atresia or Ebstein’s anomaly, and pulmonic atresia or stenosis. Ductal-independent lesions result in pulmonary and systemic mixing leading to deoxygenating of the arterial blood; hence cyanosis. These lesions include: truncus arteriosus, transposition of the great arteries, and total anomalous pulmonary venous connection. In these conditions, mixing typically occurs through an atrial septal defect (ASD), patent foramen ovale (PFO), or ventricular septal defect (VSD). Differentiating Cyanotic Congenital Heart Lesions Ductal-dependent lesions Tetralogy of Fallot Tricuspid atresia or Ebstein’s anomaly Pulmonic atresia or stenosis Ductal-independent lesions Truncus arteriosus Transposition of the great arteries Total anomalous pulmonary venous return Hypoplastic left heart syndrome HISTORY TAKING: KEY SYMPTOMS A thorough cardiac history including prenatal, perinatal and family history should be taken. For older children history of a heart murmur, respiratory distress and exercise intolerance is helpful. Important flags on history include: Cyanosis - Timing and location (peripheral or central) of cyanosis, clubbing - Refractory cyanosis if fails to improve with oxygen therapy Fainting or cyanotic spells - Cyanosis occurring with exertion, emotions, and/or bearing down, indicative of dynamic and increasing right ventricular outflow tract obstruction, which results in increased rightto-left shunting through intracardiac shunt (VSD, ASD) Exercise Intolerance - History of exercise intolerance, including dyspnea and/or diaphoresis on minor exertion, or palpitations with exertion Gestational History and Family History - Prenatal screening is important as many genetic syndromes are associated with congenital cardiac malformations - Maternal illness: diabetes, rubella, teratogenic medications. - More recently, mothers or fetuses at-risk for congenital cardiac malformations usually require a fetal echocardiogram to investigate for cardiac defects - Family history of congenital cardiac disease is important, as more and more individuals with congenital heart disease are living into their reproductive age. Please note that an approach to cardiac history taking is available on LearnPediatrics.com PHYSICAL EXAMINATION: KEY FINDINGS A thorough physical examination should be performed, including complete cardiac and respiratory examination. Of note, important findings include: Inspection - Dysmorphic features of genetic/congenital malformations (ie. Down’s syndrome is associated with endocardial cushion defects, Turner’s syndrome associated with coarctation of aorta) - Look for the signs of peripheral (nail beds) and central (mucous membranes) cyanosis - Differential cyanosis (oxygen saturation in lower limbs < upper limbs) Cardiac Examination - Heart rate, pulse oximetry, palpate central and peripheral pulses and/or measure blood pressure in upper and lower extremities - Palpate for loud heart sounds, parasternal heave, apical impulse and thrill. - Auscultate for abnormal (ie. single or widely split S2) and extra heart sounds and murmurs (Grade, timing, location, radiation, intensity and maneuvers) - Signs of heart failure: parasternal heave and palpable P2 (pulmonary hypertension), elevated JVP, hepatomegaly, and peripheral edema (right-sided failure), displaced apical impulse (enlarged LV) Respiratory Examination - Observe for signs of respiratory distress like tachypnea, dyspnea (ie. accessory muscles, paradoxic diaphragm) and hypoventilation - Palpate for asymmetric diaphragmatic expansion, and in older children percussion for consolidation, pleural effusions, and pneumothorax - Auscultate for air entry, listen for rales/crackles (consistent with effusions and/or consolidation) - Congestive heart failure: Poor air entry and dullness to percussion at the bases (pleural effusion), along with basal rales/crackles (pulmonary edema) Please note that an approach to murmurs is available on LearnPediatrics.com INVESTIGATIONS FOR CYANOTIC CONGENITAL HEART DISEASE If cyanotic heart disease is suspected, the following investigations are available for confirmation of underlying diagnosis: Electrocardiogram (ECG) Right ventricular hypertrophy: transposition of great arteries, total anomalous pulmonary venous return, tetralogy of fallot, double-outlet right ventricle, PPHN Left or bi-ventricular hypertrophy: truncus arteriosus, transposition of great arteries, pulmonary or tricuspid atresia, asplenia syndrome Right bundle branch block: Ebstein’s anomaly Chest X-ray (CXR) Chest x-ray will demonstrate cardiac size and pulmonary vascularity. Cyanotic cardiac lesions with increased vascularity include simple TGA, TAPVR, DORV, and polysplenia syndrome. Cyanotic cardiac lesions with decreased vascularity include TOF, complex TGA, Ebstein’s anomaly, pulmonary and tricuspid atresia, and asplenia syndrome. Certain unique chest x-ray findings might be more helpful and include: “Egg-shaped” heart transposition of great arteries “Snowman” in total anomalous pulmonary venous return “Boot-shaped” heart in Tetralogy of Fallot Extreme cardiomegaly can occur in Ebstein’s anomaly Hyperoxia Test: Hyperoxia test is performed to demonstrate the response of the neonate’s arterial PaO2 to 100% oxygen. Typically, if the cause for cyanosis is non-cardiac, the arterial PaO2 will increase to ≥ 100 mmHg on exposer to 100% oxygen. However, if there is a cardiac cause for cyanosis, the PaO2 will remain below 100mmHg. Pre-ductal and Post-ductal Pulse Oximetry: Pulse oximetry from the upper (pre-ductal) and lower/umbilical (post-ductal) arteries can help identify a right-to-left shunt occurring through the ductus arteriosus. Typically, a difference of > 5% in the presence of normal oxygen saturation in upper limbs pre- and post-ductal measurements suggests a right-to-left shunt. Furthermore, differential cyanosis (ie. cyanotic in the lower extremities) is a manifestation of an extreme pre- to post-ductal PaO2 gradient, which can occur in PPHN with interrupted aortic arch, and coarctation of the aorta. Of interest, reverse cyanosis in the upper but not lower extremities could be a manifestation of an aortic coarctation or interruption with concurrent transposition of the great arteries. Transthoracic Echocardiogram (TTE) A transthoracic echocardiogram will accurate identify a congenital cardiac lesion causing the cyanosis. For example, two-dimensional echocardiogram can identify anatomical lesions like septal defects (atrial or ventricular), tetralogy of Fallot etc. The additional spectral Doppler can determine degree and direction of shunt and the grading of outflow tract obstructions. Additional Modalities Additional imaging modalities include cardiac catheterization and cardiac magnetic resonance to further clarify abnormal anatomy in preparation for cardiac surgery. SUMMARY OF CYANOTIC CONGENITAL HEART DEFECTS Here, we present a brief summary of the pathophysiology of common congenital heart defects. For an in-depth summary of congenital heart defects, consider reading Pediatric Cardiology for Practitioners by Myung K. Park. Transposition of the Great Arteries In transposition of the great arteries, the aorta arises from the right ventricle while the main pulmonary artery arises from the left ventricle. The systemic and pulmonary circulations run in parallel with significant cyanosis occurring shortly after birth. As a result, most newborns are dependent on a patent ductus arteriosus or ventricular septal defect (30-40% of cases) to maintain appropriate oxygen saturations. Most of the children with this condition now a day managed with arterial switch operation (aorta is connected to left ventricle and pulmonary artery to right ventricle) along with correction of associate lesions like closure of ventricular septal defect. Tetralogy of Fallot Tetralogy of Fallot is the constellation of four congenital cardiac lesions, including: right ventricular outflow tract obstruction (pulmonary infundibular stenosis), overriding aorta, ventricular septal defect, and right ventricular hypertrophy. There is a resulting variable right-toleft shunt of deoxygenated blood, due to the dynamic right ventricular outflow tract obstruction. Typically, infants present with cyanosis shortly after birth or cyanotic spells “tet spells” with crying, exertion resulting in frequent squatting. Importantly, a variation of Tetralogy of Fallot with pulmonary atresia will result in severe cyanosis immediately at birth. Most of the patients are surgically repaired around 4-6 months of age. Total or partial Anomalous Pulmonary Venous Return (TAPVR or PAPVR): In this condition, the pulmonary veins have abnormal connection or drainage into systemic veins resulting in volume overloading of the heart. Infants with TAPVR (all the pulmonary veins) may present with symptoms of heart failure or with severe cyanosis and pulmonary hypertension if obstructed. Anomalous pulmonary venous drainage can be divided into: Supracardiac (superior vena cava), cardiac (coronary sinus, right atrium), infracardiac (portal vein, hepatic vein, inferior vena cava), and a mixed drainage. Obstructed TAPVR requires urgent surgery. Tricuspid or Pulmonic Atresia The presence of tricuspid or pulmonic atresia typically requires a concurrent abnormality, such as an atrial or ventricular septal defect and/or patent ductus arteriosus for survival. Infants present with cyanosis and require surgical treatment. Ebstein’s Anomaly Ebstein’s anomaly involves the displacement of the septal and posterior leaflets of the tricuspid valve into the right ventricle, resulting in severe tricuspid regurgitation and functional right ventricular hypoplasia. There is a resultant dilatation of the right atrium, which can be seen on chest x-ray. Treatment is usually surgical depending upon the severity. Hypoplastic Left Heart Syndrome Hypoplasia of the left ventricle results in a small and non-functional left ventricle to sustain systemic circulation. Although tolerated in utero due to right ventricular compensation, the hypoplastic left ventricle is unable to maintain adequate systemic flow after birth. Usually ductus arteriosus is kept patent with prostaglandins till staged surgical palliation (Norwood operations) is performed and followed by separation of systemic and pulmonary circulation with Fontan operation. Truncus Arteriosus A persistent truncus arteriosus arises from the early fetal truncus, a single arterial trunk that gives rise to both the pulmonary and systemic circulation. If this single arterial trunk fails to separate, there remains a single outflow tract from the right and left ventricles, with a truncal valve. This is usually associated with VSD. Double-Outlet Right Ventricle A double-outlet right ventricle simply means both the aorta and pulmonary artery arise from the right ventricle, and the left ventricle outlet is through a large VSD. Patients may present with either heart failure or severe cyanosis. This condition is repaired with different types of surgery depending upon other associated lesion. Heterotaxy: Asplenia (right atrial isomerism) and Polysplenia (Left atrial isomerism) Syndromes Heterotaxia refers to the failure of differentiation between the right- and left-sided organ, which results in not only complex cardiac lesions but also many other non-cardiac malformations especially abdominal organs. These two syndromes are most accurately summarized and compared in the following table: Cardiovascular Spleen Lungs Liver ASPLENIA Bilateral SVC, anomalous pulmonary venous return, absence of the coronary sinus, endocardial cushion defect, pulmonary stenosis, transposition of great arteries, Splenic agenesis (**infection risk**) Trilobated lungs with eparterial bronchus Midline liver, independent hepatic vein on the opposite side of the IVC Gastrointestinal Right-sided or midline stomach, gastrointestinal malrotation Eisenmenger’s Syndrome: POLYSPLENIA Interrupted inferior vena cava with azygos continuation, pulmonic stenosis, aortic stenosis or coarctation, endocardial cushion defects Multiple but nonfunctional splenules Bilobated lungs with hyparterial bronchus Usually normal but may be Midline, Direct drainage of hepatic veins into atrium Malrotation or biliary abnormalities Eisenmenger’s syndrome is cyanosis due to right-to-left shunting intracardiac shunt. Acyanotic lesions (such as atrial or ventricular septal defects) are typically able to maintain appropriate oxygen saturations despite a left-to-right shunt. However, persistent left-to-right shunting over time causes pulmonary overcirculation resulting in increased pulmonary vascular resistance. Pulmonary vascular resistance increases to a point where right-sided pressures become greater than left-sided pressures leading to right-to-left shunt (shunt reversal) causing cyanosis. Signs of Eisenmenger’s syndrome, apart from central cyanosis, include the findings of pulmonary hypertension and right-sided heart failure such as right ventricular heave, palpable P2, pulmonic or tricuspid regurgitation, elevated JVP, hepatic congestion/palpable liver, and peripheral edema. MANAGEMENT OF CYANOTIC CONGENITAL HEART DISEASE The following is a brief summary of acute and long term management of cyanotic congenital heart disease. For an in-depth approach to managing specific congenital lesions, consider reading Pediatric Cardiology for Practitioners by Myung K. Park. Medical Management Apart from supplemental oxygen, medical management of cyanotic heart diseases includes prostaglandin E1 infusion in ductal-dependent lesions to maintain a patent ductus arteriosus. This will usually serve as a bridging therapy towards intervention or cardiac surgery. Atrial Septostomy In cases of life-threatening cyanosis and/or pulmonary hypertension, a balloon atrial septostomy is done by threading a wire through the foramen ovale, and inflating a balloon to open the foramen ovale. This allows adequate mixing in the case of transposition of the great arteries, and relieves right-sided pressures in the setting of pulmonary hypertension. Cardiac Surgery A number of surgical procedures can either correct congenital anomalies, or stabilize an infant with congenital abnormalities. These palliative procedures can often serve as long-term options, and include Blalock-Taussig shunt, Glenn procedure, and others. GLOBAL REFERENCES 1. Park, MK. Pediatric Cardiology for Practitioners. 5th Edition. Philadelphia: Mosby Elsevier; 2008. ADDITIONAL REFERENCES 1. Park, MK. The Pediatric Cardiology Handbook. 4th Edition. Philadelphia: Mosby Elsevier; 2010. 2. Rose V,Izukawa T, Moës CA. Syndromes of asplenia and polysplenia. A review of cardiac and non-cardiac malformations in 60 cases withspecial reference to diagnosis and prognosis. Br Heart J. 1975 August; 37(8): 840–852. 3. Marton T, Cesko I, Hajdu J et al. Heterotaxy syndrome, analysis of 13 cases and review of the literature. Orv Hetil. 2002 Feb 10; 143(6):299-301. 4. Cesko I, Hajdu J, Marton T, Tarnai L, Zs Toth E. Familial heterotaxy syndrome. Case report and review of the international. Literature. Orv Hetil. 1998 Nov 15; 139(46):27758. 5. Leite, J. Asplenia-polysplenia syndromes. 2006 [updated 2006 Jan 18; cited 2012 Dec 1]. Available from: www.thefetus.net. 6. Vonder Muhll I. Cyanotic Heart Disease and Eisenmenger’s Syndrome. Edmonton: The University of Alberta; 2011 [updated 2011 Sep 9; cited 2012 Dec 1]. Available from: www.medicine.med.ualberta.ca.