* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download Regulation of Cardiac Output Effects of Autonomic Antagonists on
Management of acute coronary syndrome wikipedia , lookup
Heart failure wikipedia , lookup
Jatene procedure wikipedia , lookup
Cardiothoracic surgery wikipedia , lookup
Electrocardiography wikipedia , lookup
Cardiac contractility modulation wikipedia , lookup
Coronary artery disease wikipedia , lookup
Arrhythmogenic right ventricular dysplasia wikipedia , lookup
Cardiac surgery wikipedia , lookup
Quantium Medical Cardiac Output wikipedia , lookup
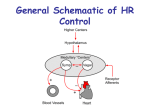
Regulation of Cardiac Output • Cardiac Output = Heart Rate x Stroke Volume • Cardiac output is regulated by changing HR and SV • SV is regulated both by changes in cardiac pumping performance (e.g. Emax), afterload (e.g. TPR) and preload (e.g. blood volume) • Major control of HR is via autonomic regulation of the SA node: • Sympathetic Nervous System (SNS) increases automaticity (exercise, emotional stress) • Parasympathetic Nervous System (PSNS) decreases automaticity (sleep) • HR control is typically achieved by reciprocal action of both SNS and PSNS Effects of Autonomic Antagonists on Heart Rate ← Intrinsic HR Parasympathetic tone (blocked by muscarinic receptor agonist, atropine) normally dominates sympathetic tone (blocked by β-adrenergic receptor blocker, propranolol) at rest Cardiac Parasympathetic Nerve Pathways • Cardiac parasympathetic fibers originate in the medulla oblongata at the dorsal motor nucleus of the vagus • Efferent vagal fibers pass via neck to synapse with postganglionic cells on epicardium near the SAN and AVN • Right vagus nerve → SAN mostly, inhibits SAN firing • Left vagus nerve → AVN mostly, delay AV conduction or even cause complete heart block • Neurotransmitter is ACh, but the SAN and AVN are rich in acetylcholinesterase • ACh directly activates KACh current so effects are rapid (<100 ms) because no second messenger is needed but transient (due to ACh-esterase activity) • Thus vagal activity can regulate HR on a beat to beat basis • PSNS is faster than SNS because no second messenger is needed 1 Vagal Effects on HR • When vagus nerves are stimulated for just a few seconds HR decreases rapidly a reaches steady state within two beats • Vagal stimulation has a much greater effect than SNS stimulation because ACh suppresses release of norepinephrine from sympathetic nerve terminals Cardiac Sympathetic Nerve Pathways • Cardiac sympathetic fibers originate in lower cervical and upper thoracic segments of the spinal cord • Pre- and post-ganglionic neurons synapse in the stellate and middle cervical ganglia • SNS and PSNS nerves join to form a mixed efferent cardiac plexus • SNS nerves approach along the vessels and spread out over epicardium of atria and ventricles, penetrating the myocardium along the intramural coronaries, which they also innervate. SNS Effects on Heart • Myocardial adrenergic receptors are primarily β1, β2 and β3 agonized by isoproterenol and blocked by propranolol • Left and right SNS nerves distribute differently: Left have more effect on contractility, right more on HR • SNS stimulation effects take 15 seconds to activate and 2 min to deactivate • This is because of signaling and the fact that norepi is not released as rapidly or inactivated as quickly • PDEs take time to degrade cAMP the main second messenger 2 SNS and PSNS Cellular Mediators Higher Centers Affect Cardiac Function • • • • Frontal lobe, motor and pre-motor cortex,… (excitement and exercise) Thalamus (tachycardia) Hypothalamus (temperature responses → HR and TPR) Stimulation of parahypoglossal region of medulla activates cardiac SNS and inhibits PSNS pathways • Dorsal medulla has distinct tachycardia and bradycardia sites (ipsilateral) Baroreflex also Affects HR • The relationship between HR and MAP is mediated by reciprocal changes in SNS and vagal firing • Below 100 mmHg high HR is dominated by SNS fibers • Above 100 mmHg vagus dominates 3 Bainbridge Reflex (1915) • At low HR right atrial filling increases HR (blocked by cutting vagi) • At high HR RA filling decreases HR due to baroreflex • ANP is released with atrial stretch having string diuretic and natriuretic effects on the kidneys • Atrial stretch sensing cells primarily in venoatrial junctions. They also activate a neurally mediated reduction in vasopressin (ADH) from posterior pituitary acting to increase urine volume Heart Failure • Activation of renin-angiotensin-aldosterone (RAAS) system • Salt and water retention due to aldosterone release from the adrenal cortex • Ventricular expression of ANP and increased ANP secretion act to attenuate fluid and salt retention • Sympathetic activation • Chronic activation of beta adrenergic receptors downregulates and desensitizes them and blunts adrenergic responsives Respiratory Effects on HR • Respiratory Sinus Arrhythmia • HR increases during inspiration (partial SNS effect) • HR decreases during expiration (increased vagal activity) • Vagal effects dominate • Thoracic pressure influences venous return and atrial filling pressure • Lung stretch receptors also activate cardiac vagal center in the medulla • Respiratory activity affects HR via peripheral arterial chemoreceptors • Decreased oxygen saturation at carotid chemoreceptors affects HR 4 Control of Contractility • • • • • Frank Starling Mechanism and Anrep Effect are mediated by stretch Extrinisic factors: circulating catecholamines, SNS lusitropy and inotropy Force-frequency relation Adrenomedullary hormones • Epinephrine • Thyroid hormones increase Ca uptake and BAR sensitivity • Insulin-positive inotropic effect • Glyocogen-positive inotropic and chronotropic effects • Oxygen and CO2 • Decreased pO2 and increased pCO2 decrease contractility Autonomic Nervous System Target Sympathetic (adrenergic) Parasympathetic (muscarinic) Cardiac output SA node: heart rate Atrial contractility Ventricular contractility β1, (β2): increases β1, (β2): increases β1, (β2): increases β1, (β2): increases contractility increases automaticity β1: increases conduction increases automaticity M2: decreases M2: decreases M2: decreases --- AV node M2: decreases conduction Atrioventricular block b-adrenergic signaling and cardiovascular disease (CVD) • Mediator of sympathetic autonomic responses of the heart • Fight or flight “response” to stress • Adaptive response to impaired function in CVD • Beta blockers used by 10’s of millions • • • of Americans (5th most widely prescribed class of medicines) Prescribed for high blood pressure, heart failure, cardiac arrhythmia, coronary heart disease Congestive heart failure affects 4.9 million Americans Coronary heart disease affects 13 million Americans (American Heart Association, Heart Disease and Stroke Statistics — 2005 Update) 5 b-Adrenergic Regulation of ExcitationContraction Coupling Saucerman, JJ et al., J Biol Chem 278: 47997 (2003) Activation of cAMP-Dependent PKA Epinephrine, Norepinephrine G c g R GTP D C C A D R C AC sAC P Na Ca2+ Cl Ga + - K+ Ca2+ PKAR PKAR GDP SOS Raf1 Gg ACI, V, VI Caln ACI PLC PIP2 PKC ACIX DAG IP3 P eNOS eNOS Signaling VASP RhoA Pln P P PTP Actin Polymerization P Rap1 Ca2+ Rho Kinase CRP 4 Ras Ga-S Gb CamK4 PKAC P Glycogen Addu TnnI GSK3 DARPP32 P cin MyosinII/ RLC Regulation of Cytoskeleton GYS APC Glycogen Synthesis Protein Retention P ATF1 P IP3R Ca2+ ERK P CREM P CREB CBP P P C 2007-2009 SABiosciences.com RyR K D EL R PPtase1 MEK1,2 BRaf Endothelial Cell Regulation Oncogenesis P GRB2 CamKs PKAC PHK Myocardial Contraction R T K C SHC Ga-S PKA Glycolysis Growth Factors GTP Lipolysis PO Ucn2 C R H R ACII, IV, VII Reduction in Rhodopsin Phosphorylation GRK1/7 Desensitization A K A P HSL Gg Calm P Ucn C Gb GDP Ga-I GTP PDE P Cell Survival Glucose-1- N GP C R cAMP BAD P PYG N C Ga-S AMP Metabolic Energy 14-3-3 Neurotransmitters, Hormones, Stress, Inflammatory Stimuli Ca2+ C N G ATP HCO 3 P Ca2+ SCn, KCn, ClCn, CaCn Ga-Q, Ga - Na + Cl + K Netrin1 N Gcg N 14-3-3 NFAT APC Regulation NF-kB P Gene Expression Cell Survival Elk1 P b-Adrenergic Regulation of E-C Coupling in Rabbit Model [Ca]i Experiments Iso Ctrl Ginsburg KS, Bers DM J Physiol. 556:463 (2004) VM Ctrl Iso APD90 ↓10%: Sanguinetti MC, et al. Circ Res. 68:77 (1991) Base model: Puglisi JL, Bers DM. AJP 281:C2049 (2001) 6 Modulation of Calcium Transients 1 µM Isoproterenol @ 10 sec Increased inotropy and lusitropy with PKA phosphorylation of LCC, PLB, RyR, and TnI. Saucerman JJ and McCulloch AD (2004) Prog Biophys Mol Biol, 85:261-78 Experimental data from: Xiao, R. P. et al. J Biol Chem 269, 19151-6 (1994). In silico KO’s reveal functional roles of PKA targets Single target phosphodisruption • PLB disruption does not restore t80 • PLB and LCC anti-cooperative for t80 • No sig. role for TnI in Ca dynamics Single target phosphorylation • Big contractility increase from PLB, LCC phosphorylation • No increase in systolic Ca for RyR phosphorylation • Apparent increased relaxation w/ LCC phosphorylation Saucerman and McCulloch, Prog Biophys Mol Bio 85: 261 (2004) Congestive Heart Failure Control CHF Sham β1-AR: -75% 8 wk 16 wk SERCA: -30% CHF Sham Oudit, G. Y., et al. (2003). Circulation 108(17): 2147-52. Zarain-Herzberg, et al. (1996). Mol Cell Biochem 163-164: 285-90. Sjaastad, I., et al. (2002). Acta Physiol Scand 175(4): 261-9. NCX: +55% 7 Congestive Heart Failure Normal 7e-4 5e-4 4e-4 3e-4 2e-4 0.1 300 6e-4 Calcium (mM) Calcium (mM) Ca2+ (mM) CHF 7e-4 6e-4 Systolic Calcium (% of control) 0.7 5e-4 4e-4 3e-4 250 Normal HF 200 150 100 2e-4 50 1.E-04 1e-4 1e-4 25 26 27 28 Time (sec) 29 30 25 26 27 28 29 30 Time (sec) 1.E-03 1.E-02 1.E-01 1.E+00 Isoproterenol (µM) Holt, ET et al. (1998) J Mol Cell Cardiol 30(8): 1581-93 Xiao RP et al. (2003) Circulation 108(13): 1633-9 8