* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download Critical Care in the Cardiac Patient
Survey
Document related concepts
Remote ischemic conditioning wikipedia , lookup
Hypertrophic cardiomyopathy wikipedia , lookup
Arrhythmogenic right ventricular dysplasia wikipedia , lookup
Coronary artery disease wikipedia , lookup
Cardiothoracic surgery wikipedia , lookup
Electrocardiography wikipedia , lookup
Heart failure wikipedia , lookup
Management of acute coronary syndrome wikipedia , lookup
Jatene procedure wikipedia , lookup
Cardiac contractility modulation wikipedia , lookup
Echocardiography wikipedia , lookup
Heart arrhythmia wikipedia , lookup
Dextro-Transposition of the great arteries wikipedia , lookup
Transcript
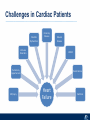
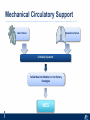
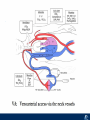
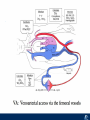
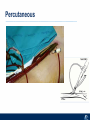
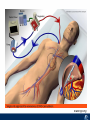
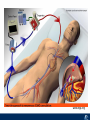
Critical Care in the Cardiac Patient Mark Joseph, MD Carilion Cardiothoracic Surgery Objectives • Identify indications for invasive hemodynamic monitoring in the critically ill cardiac patient • Describe key heart failure risk factors • Illustrate the rationale for mechanical circulatory support for the hemodynamically compromised critical care patient What is Critical Care in the Cardiac Patient? The Heart • Center of the being • Directly and Indirectly Controls the input and output of every organ in the body • Ancient books/scriptures describe the “heart” as the part of the body that “controls” the being • Reports of patient post cardiac transplant having the same urges as their donors • Taking care of a cardiac patient by default means taking care of every system that is associated to the heart • The entire patient • A happy heart = happy organs (patient) Challenges in Cardiac Patients Diastolic Dysfunction Coronary Disease Immune Disorders HOCM Pulmonary Hypertension MI/Injury Valvular Disease Renal Failure Heart Failure Nutrition Invasive Monitoring • What types of invasive monitoring • Who needs it • Non invasive monitoring – Does it really exist? Types of hemodynamic monitoring • • • • • Swan Ganz catheter Pulse Contour Analysis Bioimpendance Applied Fick Echocardiography Obituary: pulmonary artery catheter 1970 to 2013 “The birth of the conventional pulmonary artery catheter (fondly nicknamed PAC) was proudly announced in the New England Journal of Medicine in 1970 by his parents HJ Swan and William Ganz. PAC grew rapidly, reaching manhood in 1986 where, in the US, he was shown to influence the management of over 40% of all ICU patients. His reputation, however, was tarnished in 1996 when reports suggested that he harmed patients. This was followed by randomized controlled trials demonstrating he was of little use. Furthermore, reports surfaced suggesting that he was unreliable and inaccurate. It also became clear that he was poorly understood and misinterpreted. Pretty soon after that, a posse of rivals (bedside echocardiography, pulse contour technology) moved into the neighborhood and claimed they could assess cardiac output more easily, less invasively and no less reliably. To make matter worse, dynamic assessment of fluid responsiveness (pulse pressure variation, stroke volume variation and leg raising) made a mockery of his ‘wedge’ pressure. While a handful of die-hard followers continued to promote his mission, the last few years of his existence were spent as a castaway until his death in 2013. His cousin (the continuous cardiac output PAC) continues to eke a living mostly in cardiac surgery patients who need central access anyway.” Reasons for declining use of PAC Increased risk of mortality (24%) with use of right heart catheterization using PAC • Connors et al. JAMA. 1996 Sep 18;276(11). Thermodilution data provided may not be accurate • Phillips et al. Crit Care Res Pract. 2012 Clinicians didn’t know how to interpret data and large interobserver variability • Ilberti et al. JAMA. 1990 Dec 12;264(22). ESCAPE trial • JAMA, October 5, 2005—Vol 294, No. 13 • Movement across all ICUs away from invasive monitoring • “Not helpful so we shouldn’t use it” • Alternatives • Unfortunately most ICUs/clinicians have taken a “minimalist” approach Swan Ganz Catheter • PAC – “Gold standard” – operator dependent – Tricuspid/mitral valve insufficiency, shunt or misplacement may influence reliable cardiac output assessment – Some overcome by CCO PAC Minimally invasive • Pulse contour analysis, – Calibrated – uncalibrated • pulsed Doppler technology, • applied Fick principle, • bioimpedance/bioreactance. Minimally Invasive • Pulse contour – based on the principle that SV can be continuously estimated by analyzing the arterial pressure waveform. – severe arrhythmias may reduce the accuracy of cardiac output measurement, and that the use of an intra-aortic balloon pump precludes adequate performance of the device. “Non-invasive” monitoring • Pulse contour • Pro/cons • All “noninvasive” hemodynamic monitoring devices are compared to “Gold Standard” • How good is something when compared to something not so good? Applied Fick Principle • Partial CO2 rebreathing • Intubation/vented pts. • Assume CO unchanged between normal/rebreathing states • Stable pt. with no pulmonary shunt Minimally/Non-invasive Monitoring • Echocardiography – Non invasive – Readily available • Contractility – TAPSE • Volume Status – E/e’ Echocardiography • Cons – Not real time – Studies show unreliability of E/e’ in patients on inotropic support/exercise/decompensated HF/ischemia • • • • Tachycardia MR or MVR Annular calcification MS/AI Bioimpendence • Electrical bioimpedance uses electric current stimulation for identification of thoracic or body impedance variations induced by cyclic changes in blood flow caused by the heart beating. • Cardiac output is continuously estimated using skin electrodes or electrodes mounted on an endotracheal tube • Mathematical algorithms/modeling to measure CO. • Conflicting results—may be promising based on modifications. Integrative Model What can we use? • Look at all the variables • Sv02, urine output, acidosis (lactate) – If 2 out of 3 are abnormal likely to be issue with cardiac output • CO/CI, Filling pressures (PAD, CVP), SVR/SVRI • PA pressures, oxygenation • Echocardiography Integrative Model • Physical Exam – Hypoperfused state • • • • • • • • Cool extremities Low urine output Increasing CR Confusion Nausea/Vomiting Peripheral edema Ascites SOB Integrative Model • Labs – Increasing BUN/CR ratio – LFT – Coagulation abnormalities – BNP – Hyponatremia – Acidosis Which patients need this type of monitoring? • Current guidelines suggest selective use – Cardiogenic shock – Acute on chronic heart failure – Transplant/VAD patients – Escalating Inotropic support – Post procedure/MI with heart failure – At risk for RV/LV failure Key Factors Heart Failure • Pulmonary Hypertension – COPD – OSA – Morbid Obesity • Congenital Cardiac • Valvular abnormalities – History of RF – Endocarditis • History of heart failure Advances in Cardiac ICUs • Improved understanding of blood utilization • Use of hypothermia in decreasing metabolic demand • Treatment strategies for heart failure – Rematch Trial Mechanical Circulatory Support • • • • • Rationale Indications Contraindications Timing Devices Rationale • Based on concept of reversibility – Minimize collateral damage – Body/organ can recover minimal to moderate damage as long as it is allowed to recover and has reserve Indications • Cardiac- post heart TX, cardiac stun, Cardiac Failure • Massive PE, reperfusion lung injury • Severe Sepsis with Shock • Cardiac arrest Mechanical Circulatory Support Heart Failure Respiratory Failure Critically ill patient Failed Maximal Medical or Ventilatory Strategies MCS Types of Mechanical Circulatory Support IABP VAD MCS ECMO Artificial Heart E xtra C orporeal M embrane O xygenation E C L S xtra orporeal ife upport What is ECMO? • • • • First from 1971 Oxygenation outside the body Support heart and/or lung function Similar to bypass used in the operating room but can be used for longer periods of time How does ECMO Work? • Artificial pump used to pump blood out of the body; oxygen is added to the blood and carbon dioxide is removed before it is returned to the patient. • As the patient improves, we may decrease the work of artificial pump and let their heart and lungs do more of the work. • Physiologic goal – Improve tissue oxygen delivery – Remove CO2 – Allow normal aerobic metabolism – Allow heart and/or lung rest Traditional VA Cutdown Access Percutaneous www.nyp.org www.nyp.org First Successful Adult ECMO patient, 1971 Contraindications • • • • • • No likelihood of organ recovery Irreversible organ damage Disseminated malignancy Severe brain injury Unwitnessed cardiac arrest Aortic dissection/aortic regurgitation VAD (Ventricular Assist Devices) REMATCH Trial N Engl J Med. 2001 Nov15;345(20):1435-43. Outcomes in Advanced Heart Failure Patients With Left Ventricular Assist Devices for Destination Therapy Soon J. Park et al. Circ Heart Fail. 2012;5:241-248 Future Devices Conclusion • Cardiac patients most challenging ICU patients • Selective use of invasive hemodynamic monitoring should be employed for patients with identifiable risk factors for heart failure in a integrative model • As this patient population grows, treatment options improving