* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download Gene therapy and viral vectors - Lectures For UG-5
Survey
Document related concepts
Transcript
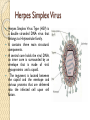
Gene therapy and viral vectors Herpes Simplex Virus Herpes Simplex Virus Type (HSV) is a double stranded DNA virus that belongs to Herpesviridae family. It contains three main structural components. A central core holds the viral DNA, an inner core is surrounded by an envelope that is made of viral glycoproteins and a capsid. The tegument is located between the capsid and the envelope and various proteins that are delivered into the infected cell upon cell fusion. The herpes family of viruses consists of three families of viruses; Alphaherpesviridae, Betaherpesviridae, and Gammaherpesviridae. Among these families are many commonly known viruses that are causative agents of many diseases, such as HSV-1 and HSV-2 for cold sores and genital warts, varicella zoster for chickenpox. Herpes virsues also tend to have latent, recurring infections in the infected organisms, where the virus remains in some part of the infected organism. Herpes family viruses are incredibly common, with at least 90% of people within the United States having been infected with at least one form of Herpesviridae. Life Cycle of Herpes Virus http://www.sumanasinc.com/webcontent/ animations/content/herpessimplex.html Herpes Simplex Viral Vector Some of the more important drawbacks Immunogenecity of the particle Packaging constraints Long term maintenance of the genetic material Random integration Cancer cell therapy with HSV HSV has many traits that make it desirable as a treatment vector; HSV can infect a wide variety of cell types, the normal replication cycle of HSV causes cells to lyse (killing cancerous cells), The HSV genome has many genes that are non-essential to replication that can be replaced with therapeutic genes, there are already many pharmaceutical options that can be used to control against unwanted replication of the virus, and the viral genome remains as an intact plasmid within the cell nucleus which protects against unwanted insertion of viral DNA into the host genome. HSV viruses were re-engineered to express cytotoxic genes or genes to stimulate immune response. The effectiveness of HSV as a treatment vector increased when used in conjunction with other cancer therapies such as radiation treatment and chemotherapy. Three strains of re-engineered HSV, designated G207, 1716, and NV1020, were put through Phase I clinical trials in 2002. There were no negative effects attributed to the virus. These strains were effective in the treatment of a variety of tumors in mice, including breast, prostate, colon, and pancreatic cancer, as well as rarer cancers. (Varghese, 2002). Phases of clinical trial Population size in clinical trials Assignment Group 1: AIDS/HIV Group2: Arthritis Group 3: Hemophilia Group 4: Hypertension Group 5: Diabetes Group 6: Hepatitis Group 7: Cancer (Any type) Group 8: Cancer (Any type) Topics to be covered in the assignment Introduction of the disease or disorder Type(s) of mutations involved Conventional treatment Type of vector The procedure of gene therapy History of clinical trials At which phase of clinical trial the treatment is undergoing? Is it commercially available? Deadline : 30 Oct