* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download Advances in Epilepsy Research - Finding a Cure for Epilepsy and
Psychedelic therapy wikipedia , lookup
Drug design wikipedia , lookup
Orphan drug wikipedia , lookup
Clinical trial wikipedia , lookup
Pharmacokinetics wikipedia , lookup
Pharmacognosy wikipedia , lookup
Psychopharmacology wikipedia , lookup
Neuropsychopharmacology wikipedia , lookup
Drug discovery wikipedia , lookup
Drug interaction wikipedia , lookup
Neuropharmacology wikipedia , lookup
Prescription drug prices in the United States wikipedia , lookup
Theralizumab wikipedia , lookup
Pharmacogenomics wikipedia , lookup
Pharmaceutical industry wikipedia , lookup
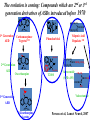
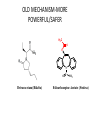
The Importance of Clinical Trials: Getting New Therapies for Epilepsy on the Market Jacqueline A. French, M.D. NYU Comprehensive Epilepsy Center The first randomized controlled trial: Lind’s treatise on scurvey • Six groups (2 patients/group): – 2pts : a quart of cider a day – 2pts: elixir of vitriol – 2 pts: vinegar – 2 pts: seawater – 2pts: mixture of garlic, mustard, spices – 2pts: oranges and lemons • Group receiving oranges and lemons fit for duty in 6 days and began to tend the other patients Why do we do clinical trials? • The American Public looks to its government for assurance that therapies developed to treat diseases are both SAFE and EFFECTIVE • The Food and Drug Administration (FDA) is charged with ensuring that safety and effectiveness are proven before a drug is put on pharmacy shelves, or before a device is marketed • They are also responsible for LABELING drugs so that the public is aware of risks and benefits • There are very strict rules that govern the conduct of clinical trials to determine safety and efficacy (effectiveness) • Without clinical trials, no new therapy would be marketed! The course of drug development • Pre-Clinical testing 10,000 Compounds 250 Get to Animal Testing 10 Reach Human Trials • Phase I – Testing in about 100 normal volunteers – Developer needs to get approval from FDA in the form of an NDA (new drug application) • Phase II/III – Tests to determine if therapy is safe and effective The course of drug development • Phase II/III (continued) – For a drug, at least 2 trials, (usually as add-on, i.e. new drug added on to existing therapy) with a control group (usually placebo(sugar pill)) • Drug must be better than “placebo” • Can see how frequent dose-related side effects are compared to placebo – It is essential to make these trials as safe and patient-friendly as possible How do new therapies get on the market? • The cost of developing a new drug is $800 million to 2 Billion and takes 12-15 years • Most drugs and devices (even if the idea comes from research labs or the National Institutes of Health (NIH) will be tested by companies that eventually will sell the product • Private sector companies need to partner with clinical researchers and doctors to perform good trials • People with epilepsy must enroll in trials in order for drugs to obtain approval from FDA Anti-seizure drugs • All available therapies only treat symptoms of epilepsy (seizures) • We now call drugs that only address seizure symptoms “Anti-seizure drugs” (ASD’s) • Most current clinical trials are for testing of ASD’s. – Almost every person with epilepsy takes at least one ASD Timeline: ASD approvals by FDA since 1990 20 Ezogabine(PotigaTM) Number of AEDs 15 Brivaracetam (RikeltaTM) Eslicarbazepine (ZebinixTM) Perampanel (FycompaTM) Clobazam (OnfiTM) Vigabatrin (Sabril Lacosamide (VimpatTM) 10 TM) Rufinamide (BanzelTM) Pregabalin (LyricaTM) Oxcarbazepine (TrileptalTM)Zonisamide (ZonegranTM) Levetiracetam (KeppraTM) Not approved Tiagabine (GabitrilTM) 5 Topiramate (TopamaxTM) Felbamate (FelbatolTM) 0 1990 Lamotrigine (Lamictal TM) Gabapentin (NeurontinTM) 1995 2000 2005 Year 2010 2015 http://www.accessdata.fda.gov 2020 Sometimes, we feel Like this… “Don’t take any of these red pills, and if that doesn’t work, don’t take any of the blue ones” DO WE NEED MORE NEW ANTISEIZURE DRUGS? • Problem with current ASDs: – Seizure control • Newly diagnosed well treated • Still 40% with therapy resistance • New ASDs over last 20 years have not substantially changed this equation! – Safety/tolerability • Some new (and old) ASDs still have important safety and tolerability problems ASD’s: How do we make progress? • Revolutionary Drugs – Drugs that work with new mechanisms never tried before – Expectation: They will control seizures that existing drugs can’t control • Evolutionary Drugs – Improve on existing drugs – Expectation: We can eliminate some of the problems/side effects of good drugs, without reducing their effect on seizures The evolution is coming: Compounds which are 2nd or 3rd generation derivatives of ASDs introduced before 1970 O NH N O C O NH NH 2 O 1st Generation AED Carbamazepinee Tegretol TM Phenobarbital CH 3CH2CH 2 CHCOOH CH 3CH2CH 2 Valproic Acid Depakote TM O O CH 2OCH 3 N N 2nd Generation C O NH AED Oxcarbazepine 2 O N O CH 2OCH 3 T2000 CH3CH2CH2 CHCONHCH2CONH2 CH3CH2CH2 Valrocemide (SPD–493) CH3CH2 * CHCONH2 CH3CH2CH CH3 H3 C O O 3rd Generation AED Valnoctamide * N O C NH 2 Eslicarbazepine Acetate Perucca et al, Lancet Neurol, 2007 Compounds which are second generation derivatives of AEDs introduced after 1990 N NH2 H Piracetam NH 2 COOH N * 1st Generation AED H Gabapentin NH 2 * Generation AED O H Precursor CNS Drug 2nd O COOH CH3 H O O NH2 Levetiracetam * N * CH3 H Pregabalin Perucca et al, Lancet Neurol, 2007 O O NH2 Brivaracetam (ucb 34714) What’s “new” in ASD’s? (Approved or close to approval) • One drug approved – Revolutionary: • Perampanel • Two drugs in late trials – Evolutionary • Rikelta (brivaracetam) • Stedesa (eslicarbazepine acetate) Perampanel • First ASD to work on excitation rather than inhibition or stabilization of membranes – “take away the kindling” rather than putting a blanket on the fire • Inhibits excitatory chemical in the brain (AMPA) • Approved for add-on treatment in partial onset seizures (adults) October 2012 Perampanel : Percent reduction in seizure frequency during maintenance phase -50 -32.13 (P=0.08) -40 Median % change in seizure frequency -39.48 (P=0.03) -30 -22.86 -20 -10 0 Placebo (n=119) Perampanel 8 mg/day (n=132) Perampanel 12 mg/day (n=130) Treatment-emergent side effects (add-on) Placebo N Treatment emergent Side effects % Perampanel 8 mg (n=133) 12 mg (n=134) (n=121) 43 6.6 6.8 19.4 Dizziness 113 9.9 37.6 38.1 Sleepiness 63 13.2 18.0 17.2 Irritability 35 5.0 7.5 14.2 Headache 54 13.2 15.0 13.4 Fall 38 6.6 9.8 12.7 Ataxia 24 0 6.0 11.9 TEAEs leading to study or study drug withdrawal Most common (≥10%) TEAEs, treatment-emergent adverse events OLD MECHANISM-MORE POWERFUL/SAFER H3 C O O * N O Brivaracetam (Rikelta) C NH 2 Eslicarbazepine Acetate (Stedesa) BRIVARACETAM (Rikelta) • Works in a similar way in the brain as Levetiracetam (KeppraTM) but much stronger in animal models • Also other activity that Keppra does not have (sodium channel blocking) • Keppra causes irritability/depression in some patientsunknown if Rikelta will have improved tolerability profile • FDA trials underway. First study very positive, second study unclear, third trial underway • First approval will be for add-on therapy for partial seizures. Other uses (eg for generalized seizures) will be explored later Efficacy of Brivaracetam (5, 20 and 50 mg/day) Add-on Treatment in Refractory Partial-Onset Epilepsy RESPONDER RATES p = 0.001 55.8% 60 40 8.0% 4/50 7.7% 4/52 7.7% 4/52 BRV5 (n=50) BRV20 (n=52) BRV50 (n=52) % Patients % Respondents 10 p = 0.002 44.2% 50 p = 0.047 32.0% 30 20 SEIZURE-FREEDOM RATES 16.7% 1.9% 1/54 10 0 0 PBO (n=54) BRV5 (n=50) BRV20 (n=52) BRV50 (n=52) ITT population: n=208; 110M, 98F; age range 16–65 y PBO (n=54) Brivaracetam Adverse Events Patients (N) Permanent study drug discontinuation Patients with ≥1 AE, n (%) Total AEs PBO BRV5 BRV20 BRV50 54 50 52 52 2 (3.7) 3 (6.0) 1 (1.9) 0 26 (52.0) 50 29 (55.8) 72 28 (53.8) 56 29 (53.7) 59 AEs reported in ≥ 5% patients Headache 4 (7.4) 4 (8.0) 2 (3.8) 1 (1.9) Somnolence 4 (7.4) 1 (2.0) 3 (5.8) 3 (5.8) Influenza 4 (7.4) 4 (8.0) 0 1 (1.9) Dizziness 3 (5.6) 1 (2.0) 0 4 (7.7) Neutropenia 1 (1.9) 4 (8.0) 2 (3.8) 0 Fatigue 2 (3.7) 0 2 (3.8) 3 (5.8) Why do we need a better Carbamazepine? • Effective drug but… – Speeds metabolism through the liver, causing: • Need for dose adjustment of other drugs that are taken simultaneously • Changes (reduction) in levels of vitamins, hormones • Increase in cholesterol levels, lipid levels • Reduction in sodium (salt) levels in the blood that can lead to problems Change in Cholesterol after removal of Tegretol or Dilantin (First to second blood draw) Tegretol Dilantin CONTROL Mintzer S. et al Effects of antiepileptic drugs on lipids, homocysteine, and C-reactive protein. Ann Neurol. 2009 Apr;65(4):448-56. Eslicarbazepine Acetate • A “third generation” Carbamazepine (TegretolTM) • Improves on second generation (TrileptalTM) – Less effect on sodium – Smoother release may produce less side effects – Does not have the same impact on the liver • Hopefully will work equally as well • Already approved in Europe as “Zebenix”. Will be marketed in US as “Stedesa”. FDA has accepted the submission Other ASD’s in development • Revolutionary: – YKP 5089 (mechanism unknown) – Ganaxolone (Neurosteroid-type positive allosteric modulation at GABAA receptor sites) – Huperzine (Naturally occurring plant alkaloid also being explored for use in Alzheimer’s disease) Is That All • There is a desperate need for – Drugs that prevent epilepsy – Drugs that modify or treat underlying disease • True antiepileptic drug – Drugs that address co-morbidities such as cognitive disturbance, mood disorder, anxiety Truly Anti-Epileptic Approaches • Anti-Inflammatory Treatments • M-Tor Inhibitors • Pre-treatment with an ASD or other therapy Targeting Inflammation • There is mounting evidence that inflammation plays an active role epilepsy • Inflammation is clearly evident in brain tissue removed from patients with epilepsy. • New paradigm: If we can target inflammation, we may be able to impact a key common mechanism and reverse the underlying cause of seizures. Vezzani A, French J, Bartfai T, and Baram T. The Role of Inflammation in Epilepsy. Nature Reviews Neurology 2011 Jan;7(1):31-40 28 What is the conclusion? • If successful, this would be the first anti-epilepsy therapy that would actually target the abnormality rather than just masking seizures • A trial of VX-765 is underway • It is likely that other antiinflammatory treatments will follow 29 M-Tor Inhibitors • Mammalian target of rapamycin (mTOR) signaling pathway regulates how brain cells grow, differentiate and multiply. • Genetic defects in the pathway can cause diseases such as Tuberous sclerosis, and cortical dysplasia (both causes of epilepsy) • In Animal models, M-Tor inhibitors can prevent or reverse epilepsy caused by these illnesses Galanopoulou AS, Gorter JA, Cepeda C. Finding a better drug for epilepsy: the mTOR pathway as an antiepileptogenic target. Clinical Trial of M-Tor Inhibitor Everolimus • A clinical trial of Everolimus (an M-Tor inhibitor) was performed in children with TS and giant cell astrocytomas. – It appeared that seizures were also improved • New trial in children/adults with TS and resistant seizures Franz DN et al. Efficacy and safety of everolimus for subependymal giant cell astrocytomas associated with tuberous sclerosis complex (EXIST-1): a multicentre, randomised, placebo-controlled phase 3 trial. Lancet. 2013 Jan 12;381(9861):125-32. Pre-Treatment: Tuberous sclerosis-Treatment with vigabatrin prior to development of epilepsy Vigabatrin Rx N=14 Standard Care N=31 Patients with Epilepsy N=22 (71%) Patients without Epilepsy N=9 (29%) Patients with intellectual Disability N=15 (48%) Patients with intellectual Disability N=0 Patients with Normal IQ N=7 (23%) Patients with Normal IQ N=7 (23%) Patients with Normal EEG N=4 (29%) Patients with Epileptiform EEG N=10 (71%) Patients with Epilepsy N=6 (42%) Patients without Epilepsy N=4 (29%) Patients with Epilepsy N=0 Patients without Epilepsy N=4 (29%) 8 pts (58%) without epilepsy and 12 pts (87%) with nl IQ Patients with intellectual Disability N=2 (14%) Patients with Normal IQ N=4 (29%) Patients with intellectual Disability N=0 Patients with Normal IQ N=4 (29%) Jóźwiak et al Eur J Paediatr Neurol. 2011 Sep;15(5):424-31. Patients with intellectual Disability N=0 Patients with Normal IQ N=4 (29%) Typical Randomized Controlled Trials vs Real Life Restricted ages No other medical Problems No psychiatric disease No pregnancy What We Know after Regulatory Trials What we know What we don’t know What do we know about AEDs at time of approval? • How the drug works in difficult to control seizures (proof that drug is better than placebo) • Side effects when used at titration rates and doses employed in trials, over short term • Safety in 1500-15,000 subjects • Drug interactions What don’t we know about AEDs at time of approval? • • • • How the drug works in other types of epilepsy How the drug works in newly diagnosed patients Comparative data vs new or old AEDs Impact at different ages – Pediatric – Elderly • • • • Best dose, titration schedule Some safety issues (including long-term) How well the drug works by itself Pregnancy effects After Approval • After approval we need “comparative effectiveness” studies – Determine which drugs will benefit which people – Unlikely that “one size fits all” • This is where government trials are needed – National Institutes of Health – Patient-Centered Outcome Research Institute (PCORI) The Epilepsy Study Consortium • Sponsored by Epilepsy Therapy Project and FACES • Group of Epilepsy Centers who work together to write protocols, bring better drugs forward, • Maintain the focus of drug development on helping people with epilepsy, NOT commercial concerns of pharmaceutical companies! The future • Need active pipeline with good compounds moving through • Need better trial designs – Shorten placebo period? – Weed out effective drugs from non-effective – Improve risk-benefit • Need patients to volunteer for clinical trials!