* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download Steps in chemical synaptic transmission and Ca2+ involvement Step
Survey
Document related concepts
Transcript
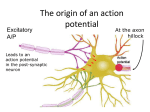
• Steps in chemical synaptic transmission and Ca2+ involvement Step 1: Neurotransmitter molecules are packaged into membranous vesicles (synaptic vesicles), which are concentrated and docked at the presynaptic terminal through the help of kinesin Step 2: The presynaptic membrane depolarizes, usually as the result of an action potential Step 3: The depolarization causes voltage-gated Ca2+ channels to open and allows Ca2+ ions to flow into the terminal Step 4: The resulting increase in Ca2+ triggers fusion of the synaptic vesicles with the presynaptic membrane Step 5: The transmitter is released into the extracellular space in quantized amounts and diffuses passively across the synaptic cleft. Step 6: Some of the transmitter molecules bind to receptors in the postsynaptic membrane, and the activated receptors trigger a postsynaptic event, usually the opening of an ion channel or the activation of a G protein–coupled signal cascade. Step 7: Transmitter molecules diffuse away from postsynaptic receptors and are eventually cleared away by continued diffusion, enzymatic degradation, or active uptake into cells. o Time from presynaptic AP to postsynaptic effect is very short, ~200 μsec for small vesicles • Neuromuscular junctions and Ach • We’re in the PNS, so axons are myelinated by Schwann cells • Each motor axon terminal has clusters of vesicles filled with ACh • AP causes vesicles to fuse with the terminal membrane • Diffuses across synaptic cleft and through the basal lamina to reach postsynaptic receptors at troughs in the muscle surface across from vesicle clusters • Neurotransmitter removal: ACh is split into acetate & choline by acetylcholinesterase in the synaptic cleft; choline then transported back to the presynaptic ending for further synthesis of ACh • Ionotropic and Metabotropic receptors • Ionotropic receptors: fast-acting, transmitter-gated ion channels • Metabotropic receptors: slow synaptic transmission; binding of neurotransmitter leads to altered concentrations of second messengers • Ach example • Nicotinic (ionotropic receptor) found at neuromuscular junctions • Can produce muscle contraction (fast EPSP) • Muscarinic (metabotropic, G protein-coupled receptor) found on smooth & cardiac muscle fibers, & on many neurons • Can produce a decrease in heart rate through increased opening of K+ channels and slow IPSP • Cholinergic, Noradrenergic, dopaminergic, serotonergic, histaminergic projection systems (primary locations of cell bodies, functions, and drugs that act on these systems) • Neuropeptides (neuroactive peptides) usually stored and released from the same neurons as one of the small neurotransmitters • Metabolically expensive; present and effective at low concentrations • Endorphins (endogenous substance with morphine-like actions) • Substance P: originally described as a smooth muscle relaxant isolated from gut, has been localized in synaptic endings in basal ganglia and DRG • Enkephalins: prominent role in pain-control circuitry • Small neurotransmitters are each stored & released by separate sets of neurons • Amino acids: glutamate, GABA, and glycine • Monoamines: acetylcholine, serotonin, histamine • Catecholamines: dopamine, norepinephrine, epinephrine • Purine derivatives: ATP Cholinergic: acetylcholine, ACh, mediates rapid, point-to-point transmission in PNS • Mainly excitatory effect, sometimes inhibitory • Cholinergic neurons of the CNS are concentrated in the brainstem, basal forebrain, and basal ganglia • Regulate general activity level of CNS neurons • • Norepinephrine affects entire CNS • NE activates mostly excitatory receptors, some inhibitory receptors • Secreted by most postganglionic neurons of the sympathetic nervous system • Fight or flight; arousal, attention, stress/panic • Main locations of neurons that (cell bodies)produce NE: Locus ceruleus, lateral tegmental area, reticular formation of the brainstem • Alertness, mood • Dopamine-containing neurons are scattered throughout the CNS • Substantia nigra – midbrain; projects to the striatum (caudate & putamen) facilitating voluntary movement • Degeneration of dopaminergic cells in the substantia nigra produces Parkinson’s disease • Ventral tegmental area – midbrain; projects to part of the forebrain (prefrontal cortex) and parts of the limbic system • Implicated in neural systems that mediate reinforcement or reward as well as aspects of drug addiction and psychiatric disorders (Schizophrenia) • Serotonergic raphe neurons associated with mood control • Histamine • Raphe nucleus, reticular formation • Sleep-wake cycle • Glutamate and GABA • Glutamate: the major transmitter for fast, brief, excitatory synaptic events in CNS • One of the transmitters at ~90% of CNS synapses • Acts on 4 major types of receptors, 1 metabotropic (G protein-coupled) and 3 ionotropic (AMPA, NMDA, and Kainate) • NMDA is responsible for some forms of long-term potentiation (LTP) • • GABA (γ-aminobutyric acid) and glycine: the major transmitters for fast, brief, inhibitory synaptic events in CNS • Glycine localized to spinal cord; GABA everywhere Any clinically relevant details • Drugs • Cocaine • blocks reuptake of norepinephrine and dopamine • Amphetamine • causes enhanced neurotransmitter release (norepinephrine and dopamine) • Cannibus • Hinders GABA inhibition more dopamine activation • LSD • Affects seratonergic system • Ecstasy • Acts simultaneously as a stimulant and a hallucinogen because of its molecular structure, which is similar to that of both amphetamines and LSD • Blocks the reuptake pumps for certain neurotransmitters, thus increasing their levels in the synaptic gap and their effect on the post-synaptic neurons’ receptors • Potentiates the effects of norepinephrine and dopamine, it is distinguished from other psychostimulants by its strong affinity for serotonin transporters • Benzodiazepines (i.e., diazepam - Valium) increase the frequency of channel opening and can increase the Cl- conductance of the GABAA receptor • Barbiturates (i.e., Phenobarbital) increase the duration of channel opening • Depression Tx • SSRIs used to treat depression (Prozac) block serotonin reuptake, prolonging serotonin effect in the brain • MAOIs prevent breakdown of serotonin • Excitotoxicity • Prolonged exposure to glutamate (through excessive release or deficient reuptake) can injure or kill neurons – excitotoxicity • Initiated by excessive Ca2+ entry through NMDA receptors • In review • Fast excitatory • PNS: ACh (nicotinic receptors) • CNS: glutamate • Fast inhibitory • GABA (GABAA, mostly in brain) • Glycine (mostly in spinal cord)