* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download Chapter 6 PPT[1].
Survey
Document related concepts
Transcript
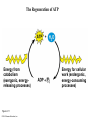
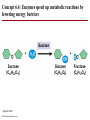
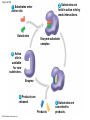
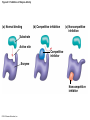
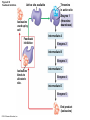
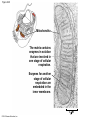
Chapter 6 An Introduction to Metabolism p. 116-134 Concept 6.1: An organism’s metabolism transforms matter and energy Metabolism is… Metabolism is an emergent property of life that arises from interactions between molecules within the cell A metabolic pathway begins with a specific molecule and ends with a product, and each step is catalyzed by a specific enzyme © 2014 Pearson Education, Inc. Figure 6.UN01 Enzyme 1 Starting molecule A © 2014 Pearson Education, Inc. Enzyme 2 C B Reaction 1 Enzyme 3 Reaction 2 Reaction 3 D Product Catabolism © 2014 Pearson Education, Inc. Anabolism Forms of Energy Energy Kinetic energy Thermal energy Heat Potential energy Chemical energy Figure 6.2 A diver has more potential energy on the platform. Climbing up converts the kinetic energy of muscle movement to potential energy. © 2014 Pearson Education, Inc. Diving converts potential energy to kinetic energy. A diver has less potential energy in the water. The Laws of Energy Transformation Thermodynamics= Isolated System Open System According to the first law of thermodynamics, the energy of the universe is constant Energy can be transferred and transformed, but it cannot be created or destroyed Chemical energy © 2014 Pearson Education, Inc. (a) First law of thermodynamics According to the second law of thermodynamics: Every energy transfer or transformation increases the entropy of the universe Heat © 2014 Pearson Education, Inc. (b) Second law of thermodynamics Biological Order and Disorder Cells create ordered structures from less ordered materials Organisms also replace ordered forms of matter and energy with less ordered forms How does energy flow into an ecosystem? How does it flow out? © 2014 Pearson Education, Inc. Concept 6.2: The free-energy change of a reaction tells us whether or not the reaction occurs spontaneously A living system’s free energy (G) is energy that can do work when temperature and pressure are uniform What is ΔG? © 2014 Pearson Education, Inc. Figure 6.5 • More free energy (higher G) • Less stable • Greater work capacity In a spontaneous change • The free energy of the system decreases (G 0) • The system becomes more stable • The released free energy can be harnessed to do work • Less free energy (lower G) • More stable • Less work capacity (a) Gravitational motion © 2014 Pearson Education, Inc. (b) Diffusion (c) Chemical reaction Exergonic and Endergonic Reactions in Metabolism Exergonic Reactions © 2014 Pearson Education, Inc. Endergonic Reactions Figure 6.6 (a) Exergonic reaction: energy released, spontaneous Free energy Reactants Amount of energy released (G 0) Energy Products Progress of the reaction (b) Endergonic reaction: energy required, nonspontaneous Free energy Products Energy Reactants Progress of the reaction © 2014 Pearson Education, Inc. Amount of energy required (G 0) Figure 6.7 G 0 G 0 (a) An isolated hydroelectric system (b) An open hydroelectric system G 0 G 0 G 0 G 0 (c) A multistep open hydroelectric system © 2014 Pearson Education, Inc. Concept 6.3: ATP powers cellular work by coupling exergonic reactions to endergonic reactions A cell does three main kinds of work Chemical Transport Mechanical Cells utilize energy coupling © 2014 Pearson Education, Inc. The Structure and Hydrolysis of ATP Adenine Phosphate groups Ribose (a) The structure of ATP Adenosine triphosphate (ATP) Energy Inorganic phosphate Figure 6.8 © 2014 Pearson Education, Inc. Adenosine diphosphate (ADP) (b) The hydrolysis of ATP Figure 6.9: How ATP drives chemical work! GGlu 3.4 kcal/mol Glutamic acid Ammonia Glutamine (a) Glutamic acid conversion to glutamine Phosphorylated intermediate Glutamic acid Glutamine (b) Conversion reaction coupled with ATP hydrolysis GGlu 3.4 kcal/mol GGlu 3.4 kcal/mol GATP −7.3 kcal/mol Net G −3.9 kcal/mol © 2014 Pearson Education, Inc. GATP −7.3 kcal/mol (c) Free-energy change for coupled reaction Figure 6.10 How ATP drives transport and mechanical work Transport protein Solute Solute transported (a) Transport work: ATP phosphorylates transport proteins. Vesicle Motor protein Cytoskeletal track Protein and vesicle moved (b) Mechanical work: ATP binds noncovalently to motor proteins and then is hydrolyzed. © 2014 Pearson Education, Inc. The Regeneration of ATP Energy from catabolism (exergonic, energyreleasing processes) Figure 6.11 © 2014 Pearson Education, Inc. Energy for cellular work (endergonic, energy-consuming processes) Concept 6.4: Enzymes speed up metabolic reactions by lowering energy barriers Sucrase Sucrose (C12H22O11) Figure 6.UN02 © 2014 Pearson Education, Inc. Glucose (C6H12O6) Fructose (C6H12O6) Figure 6.12: An energy profile of an exergonic reaction A B C D Free energy Transition state A B C D EA Reactants A B G 0 C D Products Progress of the reaction © 2014 Pearson Education, Inc. Figure 6.13: The effect of an enzyme on activation energy Free energy Course of reaction without enzyme EA without enzyme EA with enzyme is lower Reactants G is unaffected by enzyme Course of reaction with enzyme Products Progress of the reaction © 2014 Pearson Education, Inc. Figure 6.14: Induced fit between an enzyme and its substrate Substrate Active site Enzyme © 2014 Pearson Education, Inc. Enzyme-substrate complex Figure 6.15-4 2 Substrates are held in active site by weak interactions. 1 Substrates enter active site. Substrates Enzyme-substrate complex 5 Active site is available for new substrates. Enzyme 4 Products are released. Products © 2014 Pearson Education, Inc. 3 Substrates are converted to products. Effects of Local Conditions on Enzyme Activity An enzyme’s activity can be affected by: © 2014 Pearson Education, Inc. Figure 6.16: Factors that affect enzyme activity Rate of reaction Optimal temperature for typical human enzyme (37C) 0 Optimal temperature for enzyme of thermophilic (heat-tolerant) bacteria (77C) 40 80 60 Temperature (C) (a) Optimal temperature for two enzymes 20 Rate of reaction Optimal pH for pepsin (stomach enzyme) 0 1 2 3 5 pH (b) Optimal pH for two enzymes © 2014 Pearson Education, Inc. 4 120 100 Optimal pH for trypsin (intestinal enzyme) 6 7 8 9 10 Cofactors and Enzyme Inhibitors What are Cofactors? What are enzyme inhibitors? Two types © 2014 Pearson Education, Inc. Figure 6.17 Inhibition of Enzyme Activity (a) Normal binding (b) Competitive inhibition (c) Noncompetitive inhibition Substrate Active site Competitive inhibitor Enzyme Noncompetitive inhibitor © 2014 Pearson Education, Inc. Concept 6.5: Regulation of enzyme activity helps control metabolism Chemical chaos would result if a cell’s metabolic pathways were not tightly regulated How does a cell regulate its pathways? © 2014 Pearson Education, Inc. Figure 6.18a (a) Allosteric activators and inhibitors Allosteric enzyme with four subunits Regulatory site (one of four) Active site (one of four) Activator Active form Stabilized active form Oscillation Nonfunctional active site © 2014 Pearson Education, Inc. Inactive form Inhibitor Stabilized inactive form Figure 6.18b (b) Cooperativity: another type of allosteric activation Substrate Inactive form © 2014 Pearson Education, Inc. Stabilized active form Feedback Inhibition In feedback inhibition, the end product of a metabolic pathway shuts down the pathway WHY is it important? © 2014 Pearson Education, Inc. Figure 6.19 Feedback Inhibition Active site available Isoleucine used up by cell Threonine in active site Enzyme 1 (threonine deaminase) Intermediate A Feedback inhibition Enzyme 2 Intermediate B Enzyme 3 Isoleucine binds to allosteric site. Intermediate C Enzyme 4 Intermediate D Enzyme 5 End product (isoleucine) © 2014 Pearson Education, Inc. Figure 6.20 Mitochondria The matrix contains enzymes in solution that are involved in one stage of cellular respiration. Enzymes for another stage of cellular respiration are embedded in the inner membrane. 1 m © 2014 Pearson Education, Inc.