* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download Fission and Fusion of Atomic Nuclei
Inertial confinement fusion wikipedia , lookup
Muon-catalyzed fusion wikipedia , lookup
Nuclear fission product wikipedia , lookup
Valley of stability wikipedia , lookup
Nuclear fission wikipedia , lookup
Nuclear binding energy wikipedia , lookup
Nuclear fusion wikipedia , lookup
Nuclear transmutation wikipedia , lookup
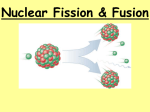
Fission and Fusion of Atomic Nuclei I. Nuclear Fission • Fission - The splitting of the nucleus into fragments (division) • Uranium-235 is struck by a neutron and forms Ba-141, Kr-92 , and additional neutrons. Neutron II. Chain Reaction • Chain reaction – Nucleus captures a neutron and splits into fragments and produces three neutrons – Products start a new reaction • Critical mass – The minimum mass required to support a self-sustaining chain reaction III. Nuclear Fusion • Fusion - combining atomic nuclei to produce a nucleus of greater mass • the nuclei of lighter elements (such as hydrogen) are fused together at extremely high temperatures and pressures to form heavier elements (such as helium) • Fusion reactions release more energy than fission reactions • Requires extremely high energies to initiate and sustain • Sun is powered by fusion euterium ritium Question Time • What is fission? • What products are formed in the fission of uranium? • What is a chain reaction? • What is critical mass? • What is fusion? • Where do we find fusion? IV. Nuclear Binding Energy • The energy required to break a nucleus into its individual protons and neutrons • Energy released in a nuclear reaction is much greater than in chemical reactions V. Mass Defect • The mass of the nucleus is always less than the sum of the masses of individual protons and neutrons. • Mass defect -The difference in mass between a nucleus and its component protons and neutrons • The difference in mass has been converted to energy • The energy can be calculated using E=mc2 Question Time • What is the nuclear binding energy? • Which releases much more energy nuclear or chemical reactions? • What is the mass defect? • What equation can be used to calculate the mass defect? VI. Nuclear Reactors • The purpose of nuclear reactors is to keep the chain reaction going without letting it get out of control Diablo Canyon San Onofre Fukushima, Japan VII. Nuclear Bombs Atomic Bomb • Uses fission • Uses enriched uranium-235 or plutonium • Nagasaki and Hiroshima • May 1945 trial run to Trinity (the first atomic bomb tested-plutonium) in New Mexico Hydrogen Bomb • Uses fission and fusion • 1000 time more powerful than atomic bomb • Uses deuterium 2H and tritium 3H • October 30, 1961, Tsar Bomba in Novaya Zemlya used Lithium for its bomb Question Time • What is the purpose of nuclear reactors? • Which is more powerful, an atomic or hydrogen bomb? Sources • • • • • • • • • • www.pennenergy.com http://www.californiareport.org noyonews.net www.sfgate.com enformable.com www.utsandiego.com activerain.com thesportdigest.com www.dailytelegraph.com.au www.2050publications.com