* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download Water as a Brønsted acid or base
Survey
Document related concepts
Transcript
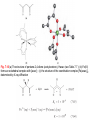
Chapter 7 Acids, bases and ions in aqueous solution TOPICS Properties of water Molarity, molality, standard state and activity Brønsted acids and bases Energetics of acid dissociation Aquated cations Amphoteric behaviour Coordination complexes: an introduction Solubility product constants Solubilities of ionic salts Common-ion effect Formation of coordination complexes Stability constants 7.1 Introduction Liquid water is approximately 55 molar H2O 7.2 Properties of water Structure and hydrogen bonding Fig. 7.2 The variation in the value of the density of water between 283 and 373 K. Fig. 7.1 Part of the structure of ordinary ice; it consists of a 3-dimensional network of hydrogen-bonded H2O molecules. The self-ionization of water If a pure liquid partially dissociates into ions, it is self-ionizing Water as a Brønsted acid or base A Brønsted acid can act as a proton donor, and a Brønsted base can function as a proton acceptor. Worked example 7.2 Manipulating equilibrium constant data Molarity: 1 M or 1 mol dm-3 contains 1 mol of solute dissolved in sufficient volume of water to give 1 L (1 dm3) of solution. Molality:1 mol of solute dissolved in 1 kg of water it is one molal (1 mol kg-1). Standard State :T = 298 K, 1 bar pressure (1 bar = 1.00 x105Pa) Activity: When concentration greater than 0.1 M, interactions between solute are significant. 7.4 Some Brønsted acids and bases The larger the value of Ka, the stronger the acid. The smaller the value of pKa, the stronger the acid. The larger the value of Kb, the stronger the base. The smaller the value of pKb, the stronger the base. Organic Acids, Carboxylic acids: examples of mono-, di- and polybasic acids Inorganic acids HCl, HBr, HI have negative pKa, where as HF is a weak acid (pKa = 3.45) Example of oxoacids include HOCl, HClO4, HNO3, H2SO4,H3PO4. The IUPAC definition of an oxoacid is ‘a compound which contains oxygen, at least one other element, at least one hydrogen bound to oxygen, and which produces a conjugate base by proton loss.’ Note that: . oxoacids may be mono-, di- or polybasic; . not all the hydrogen atoms in an oxoacid are necessarily ionizable. Phosphinic acid monobasic Inorganic bases: hydroxides NaOH, KOH, RbOH, CsOH are strong bases (LiOH is a weaker base) Inorganic bases: nitrogen bases The term ‘nitrogen bases’ tends to suggest ammonia and organic amines (RNH2), but there are a number of important inorganic nitrogen bases related to NH3 7.5 The energetics of acid dissociation in aqueous solution Hydrogen halides Fig. 7.3 The energetics of the dissociation of a hydrogen halide, HX (X=F, Cl, Br or I), in aqueous solution can be considered in terms of a cycle of steps. The significance of each step is discussed in the text. Each reaction is exothermic, with DHo values in the order HF < HCl < HBr ~ HI. H2S, H2Se and H2Te 7.6 Trends within a series of oxoacids EOn(OH)m Bell’s rule Which relates the first acid dissociation constant to the number of ‘hydrogen-free’ O atoms in an acid of formula EOn(OH)m 7.7 Aquated cations: formation and acidic properties Water as a Lewis base A Lewis acid is an electron acceptor, and a Lewis base is an electron donor. Hydration is the specific case of solvation when the solvent is water. Fig. 7.5 (a) The first hydration shell of an Na+ ion; ion–dipole interactions operate between the Na+ ion and the H2O molecules. (b) If the metal–oxygen bond possesses significant covalent character, the first hydration shell can be reasonably represented showing oxygen-to-metal ion coordinate bonds; however, there is also an ionic contribution to the bonding interaction. In practice, the character of the metal …. oxygen interaction varies with the nature of the metal ion. The configurations 7.6 and 7.7 have been established in the first hydration shell for dilute solutions of LiCl and NaCl by detailed neutron diffraction studies. In concentrated solutions, the plane of the water molecule in 7.6 makes an angle of up to 50o with the M+…O axis (Figure 7.6) implying interaction of the cation with a lone pair of electrons rather than an ion–dipole interaction. Fig. 7.6 If the plane of each water molecule in [M(OH2)6]+ makes an angle of ~ 50o with the M+….O axis, it suggests that the metal–oxygen interaction involves the use of an oxygen lone pair. Aquated cations as Brønsted acids In the aqueous chemistry of cations, hydrolysis refers to thereversible loss of H+ from an aqua species. The term hydrolysis is, however, also used in a wider context, e.g. the reaction: PCl3 + 3H2O H3PO4 + 3HCl is a hydrolysis process. Aquated cations can act as Brønsted acids by loss of H+ from a coordinated water molecule The position of the equilibrium (and thus, the strength of the acid) depends on the degree to which the OH bonds are polarized , and this is affected by the charge density of the cation. Small cations such as Li+, Mg2+, Al3+, Fe3+, and Ti3+ possess high charge densities. 7.8 Amphoteric oxides and hydroxides Amphoteric behaviour If an oxide or hydroxide is able to act as either an acid or a base, it is said to be amphoteric . The hexaaqua ion, 7.10 , may be isolated as, for example, the sulfate salt after reaction with H2SO4. The ion [Al(OH)4], 7.11, can be isolated as, for example, the Na+ salt if the source of hydroxide is NaOH. Similarly, aluminium hydroxide is amphoteric (equations 7.41 and 7.42). Periodic trends in amphoteric properties The character of the oxides of the elements across a row of the periodic table ( s- and p-blocks) changes from basic to acidic, consistent with a change from metallic to non-metallic character of the element. Elements that lie close to the so-called ‘diagonal line’ (Figure 7.8) possess amphoteric oxides and hydroxides. In group 2, Be(OH)2 and BeO are amphoteric but M(OH)2 and MO (M=Mg, Ca, Sr or Ba) are basic. Among the oxides of the p -block, Al2O3, Ga2O3, In2O3, GeO, GeO2, SnO, SnO2, PbO, PbO2, As2O3, Sb2O3 and Bi2O3 are amphoteric. Fig. 7.8 The so-called ‘diagonal line’ divides metals from non-metals, although some elements that lie next to the line (e.g. Si) are semi-metals. 7.9 Solubilities of ionic salts Solubility and saturated solutions The solubility of a solid at a specified temperature is the amount of solid ( solute ) that dissolves in a specified amount of solvent when equilibrium is reached in the presence of excess solid. The solubility may be expressed in several ways, for example: •. mass of solute in a given mass of solvent (g of solute per 100 g of water); •. moles of solute in a given mass of solvent; •. concentration (mol dm3); •. molality (mol kg 1); •. mole fraction. Fig. 7.9 The temperature-dependence of the solubilities in water of potassium iodide and sodium nitrate. The solubility of sodium chloride is essentially temperature independent in the range 273–373 K. Tabulated values of solubilities of ionic salts refer to the maximum amount of solid that will dissolve in a given mass of water to give a saturated solution. Solubilities may also be expressed in concentrations, molalities or mole fractions. Sparingly soluble salts and solubility products The solubility product,or solubility constant, Ksp The energetics of the dissolution of an ionic salt: DsolGo where F = Faraday constant = 96 485 C mol1 In solubility 7.10 Common-ion effect If a salt MX is added to an aqueous solution containing the solute MY (the ion Mn+ is common to both salts), the presence of the dissolved Mn+ ions suppresses the dissolution of MX compared with that in pure water; this is the common-ion effect. 7.11 Coordination complexes: an introduction In a coordination complex , a central atom or ion is coordinated by one or more molecules or ions ( ligands ) which act as Lewis bases, forming coordinate bonds with the central atom or ion; the latter acts as a Lewis acid. Atoms in the ligands that are directly bonded to the central atom or ion are donor atoms. In a complex: . a line is used to denote the interaction between an anionic ligand and the acceptor; . anarrow is used to show the donation of an electron pair from a neutral ligand to an acceptor. When a Lewis base donates a pair of electrons to a Lewis acid, a coordinate bond is formed and the resulting species is an adduct. The centred dot in, for example, H3B THF indicates the formation of an adduct. Fig. 7.10 (a) The structure of pentane-2,4-dione (acetylacetone), Hacac (see Table 7.7 ); (b) Fe(III) forms an octahedral complex with [acac] ; (c) the structure of the coordination complex [Fe(acac)3], determined by X-ray diffraction 7.12 Stability constants of coordination complexes In the formation of a complex [ML6]z+ from [M(OH2)6] z+ , each displacement of a coordinated water molecule by ligand L has a characteristic stepwise stability constant , k1, k2, k3, k4, k5, or k6 Thermodynamic considerations of complex formation: an introduction For a given metal ion, the thermodynamic stability of a chelated complex involving bidentate or polydentate ligands is greater than that of a complex containing a corresponding number of comparable monodentate ligands. This is called the chelate effect . 7.13 Factors affecting the stabilities of complexes containing only monodentate ligands Stabilities of complexes of non d-block metal ions of a given charge normally decrease with increasing cation size: Ca2+ < Sr2+ < Ba2+ For ions of a similar size, the stability of a complex with a specific ligand increases substantially as the ionic charge increases: Li+ < Mg2+ < Al3+ Hard and soft metal centres and ligands Pearson’s classification of hard and soft acids comes from a consideration of a series of donor atoms placed in order of electronegativity: Hard acids (hard metal cations) form more stable complexes with hard bases (hard ligands) while soft acids (soft metal cations) show a preference for soft bases (soft ligands) In aqueous solution, complexes formed between class (a), or hard, metal ions and ligands containing particular donor atoms exhibit trends in stabilities as follows: In contrast, trends in stabilities for complexes formed between class (b), or soft, metal ions and ligands containing these donor atoms are: Complex formation usually involves ligand substitution. In aqueous solution, for example, ligands displace H2O and this is a competitive rather than simple combination reaction Suppose M2+ is a hard acid. It is already associated with hard H2O ligands, i.e. there is a favourable hard–hard interaction. If L is a soft base, ligand substitution will not be favourable. If L is a hard base, there are several competing interactions to consider: Overall, it is observed that such reactions lead to only moderately stable complexes, and values of DHo for complex formation are close to zero. Now consider the case where M2+ in equation is a soft acid and L is a soft base. The competing interactions will be: