* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download Nitrogen enrichment and plant communities
Survey
Document related concepts
Overexploitation wikipedia , lookup
Island restoration wikipedia , lookup
Introduced species wikipedia , lookup
Biodiversity wikipedia , lookup
Restoration ecology wikipedia , lookup
Habitat conservation wikipedia , lookup
Ecological fitting wikipedia , lookup
Biodiversity action plan wikipedia , lookup
Theoretical ecology wikipedia , lookup
Lake ecosystem wikipedia , lookup
Nitrogen cycle wikipedia , lookup
Biological Dynamics of Forest Fragments Project wikipedia , lookup
Renewable resource wikipedia , lookup
Reconciliation ecology wikipedia , lookup
Latitudinal gradients in species diversity wikipedia , lookup
Transcript
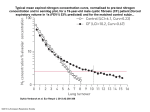
Ann. N.Y. Acad. Sci. ISSN 0077-8923 ANNALS OF THE NEW YORK ACADEMY OF SCIENCES Issue: The Year in Ecology and Conservation Biology Nitrogen enrichment and plant communities Elsa E. Cleland1 and W. Stanley Harpole2 1 Ecology, Behavior and Evolution Section, University of California San Diego, La Jolla California, USA. 2 Ecology, Evolution and Organismal Biology, Iowa State University, Ames, Iowa, USA Address for correspondence: Elsa E. Cleland, 9500 Gilman Dr. #0116, University of California San Diego, La Jolla, CA 92093, USA. [email protected] Anthropogenic nitrogen (N) enrichment of many ecosystems throughout the globe has important ramifications for plant communities. Observational and experimental studies frequently find species richness declines with N enrichment, in concert with increasing primary production. Nitrogen enrichment also reorders species composition, including species turnover through gains and losses of species, changes in dominance and rarity, and shifts in the relative abundance of particular functional groups. Nitrogen has traditionally been considered the primary limiting nutrient for plant growth in terrestrial ecosystems, but recent synthetic work suggests that colimitation by phosphorus (P), water, and other resources is widespread, consistent with theoretical predictions. At the same time, disproportionate increases in ecosystem N input are expected to exacerbate limitation by P and other resources. Similarly, synthetic research has pointed out the important role of consumers and pathogens in determining plant community structure, especially with respect to shifting resource availability. We argue here that environmental and biotic contexts, including limitation by multiple resources, herbivores and pathogens, play important roles in our understanding of plant community responses to N enrichment. Keywords: nitrogen; phosphorus; community; diversity; richness; composition; multiple resource limitation; stoichiometry Introduction Nitrogen (N) is an essential element for all forms of life, as a building block of amino acids and ultimately proteins. Although all life is bathed in N2 gas, only bacteria capable of N fixation—its conversion to available inorganic forms—can access this vast pool of N, resulting in fundamental N limitation of plants and their consumers in both aquatic and terrestrial ecosystems.1 In the early 1900s, the development of the Haber–Bosch process enabled industrial production of ammonia (NH3 ) from N2 gas, without which the global increase in crop yields and associated rise in human population growth would not have been possible.2 However, most (ca. 86%) of the reactive N created for food production is lost to the environment through a number of pathways, and does not result in human consumption.3 In addition to fertilizer production, reactive N is emitted by fossil fuel combustion. By the 1990s, anthropogenic N fixation exceeded the total amount of biological N fixation on land or in the ocean.4–6 Nitrogen enrichment of natural ecosystems as a result of human activities has had a variety of negative consequences including reductions in species richness and community structure, which will be the focus of this review. The topic of plant community responses to nutrient enrichment has been explored by excellent earlier reviews by DiTommaso and Aarssen7 and Bobbink et al.8 . This review builds on these earlier efforts by synthesizing recent literature, comparing how species richness, evenness, and functional composition of terrestrial plant communities vary along natural gradients versus with anthropogenic N enrichment. In particular, this review seeks to (1) understand the mechanisms that underlie plant community responses to N enrichment, (2) identify aspects of environmental context that modify these responses, and (3) incorporate the concept doi: 10.1111/j.1749-6632.2010.05458.x 46 c 2010 New York Academy of Sciences. Ann. N.Y. Acad. Sci. 1195 (2010) 46–61 ! Cleland & Harpole of multiple-resource limitation that may constrain community and ecosystem responses to N enrichment. Nutrients and plant community structure in natural systems Nutrient inputs in natural systems In preindustrial times, inputs of N in terrestrial ecosystems arose predominantly through bacterial N fixation, estimated to be between 100 teragrams (Tg)/year2 and 195 Tg/year,9 with an additional estimated 5 Tg N/year fixed by lightning.10 “Natural” fires (as opposed to human-caused fires) result in additional volatilization and deposition of N, a minimum of 3.2 Tg N/year is likely to be deposited as a result of “natural” fires (this number only considers fire at high latitudes).5 As N is recycled through detritus by decomposition, N is also volatilized to the atmosphere in the form ammonia (NH3 ), and related forms (NOy and NHx ). This natural rate of N deposition (as opposed to anthropogenic N deposition, which will be discussed later) has been estimated at 6.6 Tg/year in the form of NOy and 10.8 Tg/year in the form of NHx .5 Although the inorganic forms NH4 + and NO3 − are the predominant sources of plant N, a number of plants utilize amino acids and other organic forms of N, especially in highly N-limited systems (reviewed by Chapin11 and Schimel and Bennett12 ). Species also appear to differ in their preference for different forms of N and species abundances correlate to the relative availability of the form of N they prefer.13 There is great variation among ecosystems in the rate of biological N fixation, the major source of natural inputs of N. The highest rates of N fixation take place in tropical ecosystems, resulting in relatively N-rich systems14 ; as a result, other micronutrients frequently limit production in tropical systems. Legumes that have the highest rates of N fixation are frequently found to be phosphorus (P) limited, highlighting an important link between N and P cycling in terrestrial ecosystems. This observation has led to the speculation that P may be the ultimate nutrient limiting production in many ecosystems via its control over N fixation rates,15 even though short-term experiments often find that growth is stimulated when N is added. In contrast to N, P inputs to natural systems are largely physical and chemical. Root exudates and Nitrogen enrichment and plant communities mycorrhizae can increase the rate at which P is made available to plants, but this biological influence is small compared to primary importance of physical weathering. Weathering of parent material is the primary source of P for terrestrial ecosystems, estimated globally at 3 megagrams,16 with local variation from 0.05 to 5 kg P/ha year.17 Additional P is deposited in the form of dust, ash, or pollen but global estimates of these Aeolian inputs are not yet available; estimates from funnel traps range from 0.07 to 1.7 kg P/ha year.17 Ecosystem development The relative importance of N and other limiting factors changes with the development of ecosystems over geological time. Soils in the early states of primary succession often contain abundant mineral nutrients with the exception of inorganic N; deposition and biological N fixation and the accumulation of organic matter increases total N over time during both primary and secondary succession.18,19 Over geological time periods, accumulation of N is in contrast to net losses of P and other nutrients due to weathering20 . On Hawaiian islands with volcanic soils that are millions of years old the soils are P-limited, whereas soils on recent lava flows that are just a few hundred years old tend to be N limited, and soils of intermediate ages are often N&P colimited.21 Viewing successional gradients merely as transitioning from N to P limitation over time may be overly simplistic. Colimitation can occur across a gradient of soil age and also can occur on very young soils.22 Despite the general patterns of strong N limitation on early successional soils, in addition to P, Mg, and other micronutrients may also be limiting. At the other end of the soil development gradient, on geologically old soils, weathering leads to a loss of other base cations in addition P.20 Stoichimetry competition, and R∗ Theory The stoichiometry of biological organisms dictates that multiple elements are required in particular ratios simultaneously for optimal growth. Because of physiological and life history differences, species will necessarily differ in their stoichiometry.23 Organism physiology can strongly drive organism stoichiometry: vertebrate endoskeletons result in relatively low organismal N:P compared to arthropods, with their protein-based chitinous exoskeletons. Species with greater growth rates have higher P demand c 2010 New York Academy of Sciences. Ann. N.Y. Acad. Sci. 1195 (2010) 46–61 ! 47 Nitrogen enrichment and plant communities Cleland & Harpole because of greater allocation to P-rich ribosomal nucleic acids.24 In chronically nutrient limited environments, evolution should lead to adaptations that either increase the access of an organism to limiting nutrients (e.g., N fixation, phosphatase production) or decrease the demand for particular limiting nutrients: marine phytoplankton recently have been found that use nonphosphorus membrane lipids in P-limiting environments.25 Thus, because different species represent alternative evolutionary “solutions” to dealing with multiple and complex constraints, they differ in both their requirements for and ability to compete for different limiting nutrients. Because species have evolved different adaptations to multiple limiting factors, including nutrients, species show trade-offs, which can allow many species to coexist when they compete for multiple limiting nutrients.26 Nutrient availability in ecosystems is a balance between input and loss rates as well as the ability of plants to grow and deplete nutrients. The ability of plants to deplete nutrients leads to plant competition. The converse prediction is that increasing the supply of a limiting nutrient eventually makes it nonlimiting and thus no longer competed for: fertilization will cause competition to shift to a different limiting factor. Because species have trade-offs for different nutrients, changes in nutrient ratios are predicted to lead to changes in species composition and diversity.26 Increasing inputs of N or other nutrients will eventually lead to imbalanced nutrient supply and should result in strong limitation by fewer resources for which fewer species should be the best competitors (Fig. 1).27 Thus, fertilization of ecosystems can reduce the number or change the identity of the factors that were originally limiting and to which the species in that ecosystem were evolutionarily adapted, resulting in loss of diversity.27 For example, colimitation by multiple below ground resources such as N and P may allow multiple species to coexist if they trade-off the ability to acquire and use these different resources; fertilization with N should ultimately make N nonlimiting and cause greater limitation by P (fewer limiting resources) or increased limitation by light (increased importance of a different limiting factor) (Fig. 1).28 Fertilization may also homogenize environments and decreased heterogeneity should lead to loss of diversity through diminished opportunities for spatial coexistence,26 48 Figure 1. If species A–D trade off the ability to compete for nitrogen (N) and phosphorus (P), they can coexist pairwise at resource supply points in the gray triangular regions. The right-angled lines indicate resource combinations where each species has zero net growth. For example, A is the best competitor for N because it can maintain growth at the lowest levels of N. If the blue circular region represents preindustrial levels of N and P supply, N deposition (red arrow) might move the set of supply points to the red circular region. Under the new conditions of decreased N limitation, only species D would persist because it is the best competitor for limiting P. although experimental support for this mechanism has been mixed.29 Multiple resource limitation Multiple resource limitation is predicted from several lines of theory. Economic and optimal foraging theory predicts that the fitness of an organism should be maximized when it is simultaneously limited by all limiting factors.30 Nonoptimal allocation that results in unbalanced or excessive uptake of one nutrient relative to other nutrients represents a fitness cost because excess uptake must be stored or excreted; fitness should be increased by adjusting allocation efforts to allow uptake of all essential nutrients in optimally balanced ratios.30 However, the degree to which multiple limitation is observed will depend on the extent to which plants are able to deplete soil nutrient pools,31 as well as the degree to which uptake of multiple nutrients covaries.32 For example, allocation to roots allows a plant to increase access to multiple below ground nutrients c 2010 New York Academy of Sciences. Ann. N.Y. Acad. Sci. 1195 (2010) 46–61 ! Cleland & Harpole simultaneously (positive covariance), whereas allocation to symbiotic mycorrhizae may maximize P uptake at the expense of other micronutrients (negative covariance). Plants can also exert direct control over nutrient availability to minimize limitation by any single nutrient, thereby increasing the likelihood that multiple nutrient limitation will be observed. For instance, plants have evolved various adaptations to acquiring P that include variation in root architecture, phosphatase and organic acid root exudates, and mycorrhizal symbiosis.33 In addition to biological N fixation through symbiosis, plants can also exert feedback control over N cycling although effects of litter chemistry on decomposition and soil microorganism communities34,35 (but see Ref. 36). This concept of multiple resource limitation is at odds with traditional views of resource limitation stemming from von Liebig’s Law of the Minimum that there is a single resource that is the most limiting to plant growth at any given time; but this idea was developed for single species in an agricultural setting, rather than naturally diverse communities.37 Whether or not individual plants are equally limited by multiple nutrients, communities of multiple plant species should necessarily be colimited by multiple nutrients based on the mechanisms already discussed.37 Thus, to understand the impacts of N on individual plants and on communities, it is necessary to view changing N supply in the context of multiple resource limitation. Species composition along natural gradients in nutrient availability Species’ competitive trade-offs for different nutrients, as well as trade-offs for growth, reproduction, and defense are often correlated with patterns of species abundances along N gradients, and they form the basis for mechanistic theoretical solutions to species coexistence questions.38,39 Plant functional traits reflect strategies for resource capture that vary both within and among species, and can indicate important trade-offs that contribute to species coexistence. High trait dispersion (more evenly distributed trait values than expected by chance) can be indicative of interspecific competition, resulting in diverse strategies for resource capture in a community.40 Supporting this idea, trait dispersion has been shown to increase with increasing soil fertility.40 N-fixing legumes and tree species Nitrogen enrichment and plant communities (e.g., Alder spp.) are often found on early successional soils or on soils following a fire that are relatively rich in P and micronutrients and with high light availability but relatively depleted in N. Highnutrient soils favor good light competitors with high allocation to aboveground leaves and stems. Plants with various adaptations to acquiring P (e.g., root architecture, phosphatase root exudation) can be found on low P soils. Changes in nutrient supply should affect species differently. Plants that have evolved in low-nutrient environments may respond less to increases in nutrient availability than would plants adapted to highnutrient conditions.41 For instance, species adapted to low N conditions show a broad spectrum of traits corresponding to either N fixing or N conserving strategies including low N tissue, high root to shoot allocation, and correspondingly low relative growth rates.42 Species adapted to low N conditions also tend to have low extractable soil nitrate when grown in monocultures low extractable soil nitrate; this metric is indicative of the level to which a plant can deplete limiting soil inorganic N, corresponds to a plant’s competitive ability for nitrate (i.e., its R∗ ).26 Low R∗ for nitrate (better competitor for limiting N) correlates with other traits like high C:N, high root:shoot.42 Plants that are better N competitors (lower R∗ ) are also predictably more abundant in a wide variety of contexts: increasing with time along successional gradients (potential trade-off with colonization), decreasing with increasing N fertilization (N becoming less limiting), and are consistently dominant in low N old fields at multiple sites, and in community assembly experiments on low N soil.43 Plant community responses to N enrichment Anthropogenic N inputs Nitrogen (N) deposition into terrestrial ecosystems has more than doubled due to human activities, principally fossil-fuel combustion, deforestation, and agricultural intensification.2,5,44,45 These activities result in different forms of N entering the environment. For instance fossil fuel combustion results primarily in deposition of oxidized forms (NOy ). In contrast, agricultural activities result in deposition of reduced forms (NHx ). This can result in regional differences in the form of deposition. For instance, c 2010 New York Academy of Sciences. Ann. N.Y. Acad. Sci. 1195 (2010) 46–61 ! 49 Nitrogen enrichment and plant communities Cleland & Harpole Holland et al.46 synthesized wet-deposition fluxes (those occurring in precipitation) across the United States and found peak NH4 + deposition over the Midwest. In contrast, NO3 − deposition peak over the Northeastern states. In many areas, the combination of N and other atmospheric pollutants has led to acidification of soils and freshwater, causing a suite of environmental changes including longterm nutrient losses and base cation leaching,47–50 sometimes referred to as the nitrogen cascade.51 Evidence from observational studies Atmospheric N deposition has also been associated with observed declines in plant species richness (i.e., the number of species found in a given area), especially in Europe.8,52 There is variation among ecosystems in diversity decline; particularly susceptible communities are those on poorly buffered soils8 and wetlands (reviewed by Morris53 and Bedford et al.54 ). Although sensitive plant species and lichens have declined in areas with high deposition in the Western United States,55 comprehensive observational data on plant species richness responses to N deposition in North America are lacking. Even less attention has been focused on tropical regions. A recent analysis of global chemistry transport models reported that in 34 of the world biodiversity hotspots the 1990 level of N deposition was 50% greater than the global terrestrial average, with increases predicted for all but one location by the year 2050.56 This calls attention to potential negative impacts of N deposition in some of the most diverse habitats. Evidence from experimental studies Most of our understanding of how plant communities respond to nutrient enrichment comes from experiments. Nitrogen fertilization experiments have shown that N addition almost always increases primary production, but reduces species richness in terrestrial systems, and there have been a number of excellent reviews (e.g., Ref. 7) and metaanalyses,57–59 which have documented this consistent pattern. There is great variation in the degree of productivity increases and richness declines, suggesting that environmental and biotic context play an important role in modifying community responses to enrichment (see section “The importance of environmental and biotic context”). 50 Beyond richness responses, community structure has also been shown to respond to N enrichment. For instance, Collins et al.60 showed that N fertilization caused consistent changes in dominance in a variety of North American herbaceous plant communities, frequently resulting in increased dominance by one or a few species. In a recent analysis of 274 N fertilization experiments, Hillebrand et al.59 found that N enrichment tends to reduce evenness, although positive effects on evenness have also been reported.61 This analysis59 found that inherent ecosystem productivity predicted the degree of evenness change, with highly productive ecosystems experiencing the greatest decline in evenness (and hence increase in dominance) with nutrient enrichment. These observed changes in richness and evenness beg the questions: Which species are most likely to be lost, and which species may actually increase in abundance as communities are reordered? Several tentative generalizations can be made. For instance, rare species are more likely to be lost with N enrichment than abundant species, as evidenced both in an observational synthesis along gradients of N enrichment54 and in meta-analyses of N fertilization experiments.58,62 There are often clear responses at the functional group level. Xia and Wan62 found that herbaceous species had significantly greater biomass responses to N fertilization than woody species, in a global meta-analysis including 456 terrestrial plant species. For herbaceous species, Suding et al.58 found that perennial, N fixing, and native species were more likely to be lost under experimental fertilization. In a review comparing the performance of co-occurring native and invasive species, Daehler63 found that invaders frequently had a performance advantage over native species under conditions of nutrient enrichment. This finding has been particularly robust for exotic annual grasses, which increase in abundance with N addition and can drive diversity declines of forbs and N fixers.64–66 It is important to note that the spatial and temporal scales of experiments bias our understanding of plant community responses to N enrichment. Although experiments can control for many of the kinds of co-occurring changes that complicate observational studies of N deposition, most of the results of such experiments are based on small plots (often 1 m2 ). If individuals increase in size with N c 2010 New York Academy of Sciences. Ann. N.Y. Acad. Sci. 1195 (2010) 46–61 ! Cleland & Harpole enrichment, then per m2 there will be fewer individuals and decreased numbers of species because sampling area is fixed; with N enrichment the researcher is sampling a smaller effective area.67 If sampling area scaled to individual size, then the null expectation would be that increasing individual size would not be related to numbers of species if the local area was sufficiently larger than the sample area. Chalcraft et al.68 examined diversity responses to N addition at multiple spatial scales, using many of the same herbaceous plant communities analyzed by Suding et al.58 . Although the response of alpha diversity (plot scale) nearly always declined with N addition (+5% to −61%), beta diversity (species turnover among plots) responses were variable. This variation was associated with productivity; beta diversity increased in response to N enrichment at low-productivity sites (up to +22%), but decreased at high-productivity sites (up to −18% reduction). This effect on turnover in species identity among plots tended to ameliorate fertilization induced reductions in gamma diversity (i.e., species pools in control and fertilized plots integrated across whole experiments). This study points out the importance of understanding spatial heterogeneity and turnover among plant communities when scaling up from plot level experiments to regional level predictions of anthropogenic impacts. Temporal trends are also important to consider. Most experimental fertilization studies are short in duration and add nutrients in excess of demand, and at levels much higher than those experienced by natural systems due to atmospheric N deposition. One long-term gradient study added 1–10 g/m2 N to native prairie grassland in Minnesota (equivalent to 10–100 kg/ha) and found significant declines in species richness even at the lowest level of addition,69 although it took the longest time to see a significant reduction in richness at the lowest level of addition. This study is important because it shows that species are responding to cumulative N loads, that detrimental effects can result from the accrual of even small amounts of N deposition, and also suggests that short-term experiments with high N addition rates are reasonable proxies for chronic, low-level N deposition. The longest running ecological experiment, the Park Grass experiment at Rothamsted, Harpenden, UK, has received annual application of combinations of NH4 + , NO3 − , P, Na, and Mg for over Nitrogen enrichment and plant communities 150 years. Fertilization has lead to a persistent loss of species and changes in composition.70–72 The most dramatic effect of nutrient addition on species number (a decline in species number from greater than 40 to three species per plot) was largely attributable to decreased soil pH from 7.3 to 3.6 due to the acidifying effect of chronic addition of ammonium sulfate.71 This strong effect of soil acidification prompted the establishment of additional liming subplots to mitigate the impact on pH: liming the ammonium addition subplots effectively added about 15 species.71 It should be noted that communities at Park Grass are strongly colimited by multiple nutrients including N, and the greatest loss of species occurred with the addition of multiple nutrients.27,73 In contrast to species richness, the genetic diversity of Anthoxanthum odoratum populations increased in plots with greater numbers of added resources.73 This result is consistent with the hypothesis that the addition of greater numbers of limiting resources should decrease niche dimension, and thus species diversity27 ; as a consequence of species loss, niche breadth of the surviving species should increase and be reflected in the potential for greater genetic diversity of the surviving species.73 Plant community responses to multiple altered nutrients A recent meta-analysis by Elser et al.74 shows that N and P strongly colimit production across terrestrial, marine, and freshwater systems, challenging a long-standing paradigm that ecosystem production can be viewed as primarily limited by the single nutrient in lowest supply—typically thought to be N in most terrestrial systems. Addition of a nutrient makes it not limiting and thus takes away a niche axis along which some species were specialized or for which they traded off the ability to compete along some other niche axis.27 Addition of greater numbers of limiting resources is then, essentially, a “niche-destruction” experiment, which follows from Hutchinson’s75 prediction that greater numbers of niche axes, or niche dimension, should allow greater numbers of coexisting species. Harpole and Tilman27 tested this prediction experimentally and found that addition of increasing numbers of resources (e.g., combinations of N, P, K, or water), and therefore decreased niche dimension, led to a joint c 2010 New York Academy of Sciences. Ann. N.Y. Acad. Sci. 1195 (2010) 46–61 ! 51 Nitrogen enrichment and plant communities Cleland & Harpole increase in grassland community biomass and decrease in species diversity. The relationship between species richness and productivity can differ depending on the degree of N, P, and/or K limitation.76 Resource ratio theory26 makes a similar prediction: greatest species diversity should occur when multiple resources are supplied at optimal or balanced ratios; extreme or unbalanced resource ratios should correspond to reduced diversity.77,78 Orthogonal to resource ratio gradients is the total supply of resources: greater total supply, if at balanced ratios, might increase diversity by allowing greater numbers of individuals to persist, thus reducing likelihood of extinction for small populations.79 Thus, increased N deposition will change both ratios (balance) and amount of nutrients (total supply); along with these two components of fertilization. Cardinale et al. also include the causal positive effect of diversity on productivity and provide a conceptual multivariate, resource-based hypothesis for the productivity–diversity relationship.79 In summary, resource imbalance decreases both diversity and productivity (resource ratio theory); increasing total resource supply at balanced ratios tends to promote diversity and increase productivity (Species Energy theory); changes in resources that decrease diversity should additionally reduce productivity (biodiversity and ecosystem function theory); thus resource ratios, resource availability and diversity jointly and mechanistically can explain the often observed hump-shaped productivity–diversity correlation patterns.79 Changing inputs, changing limitations Despite the impressive impact human activities have had on the global N cycle, human activities have had an even larger impact on the global P cycle, increasing P flux by 400%.16 High levels of P are applied to agricultural lands but more strongly impact aquatic systems than natural terrestrial systems because P-loss pathways are directed toward waterways and the P cycle does not have a gaseous component like N that can allow widespread atmospheric deposition. Thus, alterations to the N cycle, rather than the P-cycle, have had a disproportionate effect on natural terrestrial systems because of atmospheric N deposition. In addition to high rates of N inputs to terrestrial systems that should progressively decrease N limitation and increase limitation by other nutrients over 52 time, the evidence that most systems appear to be colimited by N, P, and perhaps other nutrients, suggests that N limitation should be diminishing and there should be increasing limitation by other nutrients. Ratios of resource inputs are changing over time. Ratios of N to other nutrients (e.g., Na, K, Mg, S) in wet deposition samples from the U.S. National Atmospheric Deposition Program appear to have been increasing in recent years in the continental United States (Fig. 2). Increasing levels of N relative to other potentially limiting nutrients suggest that terrestrial systems may experience increasing limitation by nutrients other than N over time. Although N emissions in parts of the United States and Europe have plateaued or decreased, N emissions are increasing in others areas of the world, in particular in countries with growing industry such China.80 Although average total inorganic N wet deposition may not be changing significantly in the United States, the relative amounts of different forms of N do show stronger trends: NHy deposition is increasing relative to NOx wet deposition (Fig. 2). This trend has implications for altered species composition because studies have shown species’ preferences for different N forms that correlate with their abundance.13 Given that N deposition is increasing in some areas, and that other areas have already experienced about a sixfold increase in N deposition from preindustrial levels,81 we need to explore the importance of and potential limitation of other nutrients in natural ecosystems. The importance of environmental and biotic context Although species richness generally declines with anthropogenic N enrichment, there is considerable variation in this response across ecosystems (section “Plant community responses to N enrichment”). In addition to stoichiometric requirements that could constrain species and hence community responses to N enrichment (section “Plant community responses to multiple altered nutrients”), there has been a growing recognition that environmental context plays an important role in predicting variation in community responses to N enrichment. For instance, species often respond to N addition in predictable ways: for species that occurred across multiple N fertilization experiments, 10 of c 2010 New York Academy of Sciences. Ann. N.Y. Acad. Sci. 1195 (2010) 46–61 ! Cleland & Harpole Nitrogen enrichment and plant communities Figure 2. Nutrient ratio wet deposition trends from annual means of 290 continental USA National Atmospheric Deposition Program monitoring sites (excluding Alaska). Polynomial regressions suggest that historically increasing deposition rates of inorganic N relative to other nutrients are decelerating, and may reflect recent decreases in inorganic N deposition in some regions of the United States.123 Although recent rates of inorganic N (NH4 plus NO3 ) deposition, on average, show no significant increase, the ratios of NO3 to NH4 have declined, and ratios of N to other nutrients have generally increased. 20 consistently increased or decreased, but abiotic factors were also important.82 Clark et al.83 used a structural equation model to ascertain the factors that best explained the variation in species response (0–65%) in 23 fertilization experiments. Greater species loss occurred in communities with lower cation exchange capacity, colder regional temperatures, and stronger production responses to fertilization. Similarly, Stevens et al. found that high mean annual precipitation and high soil pH moderated the negative impact of N deposition on species richness across a gradient of N deposition in Great Britain.52 In this section, we summarize the importance of environmental context (in particular water and light availability) and biotic interactions (herbivores, pathogens, soil microbes) that can mod- ify community responses to N enrichment, and can also potentially reveal the mechanisms that underlie these changes (Fig. 3). Water Community responses to N enrichment may depend on precipitation, both because of the fundamental constraint of water limitation in many ecosystems84 and because water availability influences the diffusion of soil N to plant roots and N mineralization. Frequently, N addition can lead to decreased soil moisture,65,85 either because of increased water demand through increasing biomass, or through increased stomatal conductance as a consequence of increased leaf N and photosynthetic rates.86 Furthermore, N deposition rates tend to c 2010 New York Academy of Sciences. Ann. N.Y. Acad. Sci. 1195 (2010) 46–61 ! 53 Nitrogen enrichment and plant communities Cleland & Harpole Figure 3. Photographs of experimental plots under (A) control conditions or (B) N addition at a rate of 7 g N/m2 year in the form of calcium nitrate, in central coastal California (Cleland and Suding, unpublished). Notice that N addition increased production and dominance of exotic annual grasses and reduced diversity of native forbs. A summary of hypothesized mechanisms leading to declines in species richness and evenness with N enrichment follows. be correlated with rainfall levels,87,88 as do N loss rates through leaching and runoff.89 Using a global data set, Xia and Wan62 found that the percent increase in production with fertilization increased linearly with mean annual precipitation. In contrast, in a synthesis of fertilization experiments in arid or semiarid ecosystems, the absolute response of production to N enrichment increased with increasing annual precipitation, but the relative response did not change.90 The conclusion from both of these meta-analyses was that N and water colimited production across the range of ecosystems included. 54 Factorial N and water addition experiments have found different effects of their interaction on species richness. Factorial experiments are illustrative for identifying the mechanisms that might drive responses at multiple levels for organization. In an annual grassland adding N and water both increased productivity, but only N addition lowered species richness.91 In this case timing was important: N addition increased biomass and decreased light levels earlier in the growing season, resulting in a decline in short-statured species. Harpole et al.85 found a slightly different response to factorial additions of N and water in California annual grassland, where N c 2010 New York Academy of Sciences. Ann. N.Y. Acad. Sci. 1195 (2010) 46–61 ! Cleland & Harpole and water only increased productivity when added simultaneously, but N addition caused a significant decline in species richness both alone and in concert with water addition, suggesting it was not the increase in biomass that drove the richness response. Nitrogen enrichment and plant communities Light Light limitation associated with increased aboveground production is one of the primary mechanisms hypothesized to explain diversity loss in response to N enrichment.38,92 In addition, high production can result in high litter loads, limiting light availability for seedlings.93 An elegant experiment by Hautier et al.28 strongly suggested that competition for light was the driver of richness decline following nutrient enrichment. In their experimental grassland communities, the decline in species richness following fertilization (N, P, and micronutrients) was ameliorated by lights hung inside the plant canopy, which eliminated light limitation in the understory. Other recent work, however, suggests that light limitation may not be responsible for richness declines (e.g., Ref. 94). Lamb61 manipulated soil N, water, and light availability in a rough fescue prairie in Canada, and analyzed their interacting effects on species richness and evenness using structural equation models. This showed that the negative effect of N enrichment on richness was mediated largely through increased litter accumulation but not via decreased light availability. Instead other effects of increased litter (via herbivore or pathogen loads, see later sections) may have been responsible. that the combination of herbivory and fertilization had the strongest positive effects on richness with low-productivity responses to fertilization. Herbivore performance and population sizes generally show positive responses to anthropogenic N deposition, a survey of the literature found that plant N concentration generally increased and carbonbased defense compounds decreased in response to deposition.96 Nitrogen content of plant tissues is one of the best predictors of herbivore performance,97 and it has been suggested that the greater frequency of bark beetle outbreaks may be related to increasing N deposition rates.96 This recalls the theoretical concept of the “paradox of enrichment,” whereby increasing resource availability for plants may induce a population increase in their consumers, ultimately driving the consumed species near extinction.98 Hence, although herbivory rates may mediate species richness declines in response to fertilization in small-scale experiments, anthropogenic deposition could have negative consequences for diversity via increased populations of herbivores. The degree of stoichiometric mismatch between plants and herbivores is also likely to mediate the effect of herbivory on plant growth and community composition.99 As an example of stoichiometric requirements changing with trophic level, livestock production in California rangelands can be limited by forage protein. One management strategy has been to fertilize pastures with P and S, thus particularly increasing the production of N-fixing legumes.100 Herbivores Using a meta-analysis of herbivore exclosure and fertilization experiments, Gruner et al.95 showed that herbivory greatly limits the apparent productivity increase with fertilization in both terrestrial and aquatic ecosystems. In a complementary metaanalysis using many of the same studies, Hillebrand et al.59 showed that species richness and evenness responded in complex ways to manipulation of both nutrients and herbivores. Herbivores generally increased richness and evenness, both alone and in combination with fertilization. The researchers hypothesized that herbivory on dominant species may have contributed both to increased evenness and removal of biomass, hence preventing light limitation. This hypothesis was supported by the fact Pathogens Pathogens can dramatically reduce plant productivity,101 and can alter plant community composition and diversity.102 In general, N enrichment increases plant susceptibility to pathogenic fungi,103–105 although declines in susceptibility have also been reported (e.g., Ref. 106) and the effect likely varies among pathogen types.107 For example, in Sweden the parasitic fungus Valdensia heterodoxa played a key role in vegetation responses to nutrient enrichment,103 whereby the shrub Vaccinium myrtillus had increased fungal infection with N enrichment, resulting in increased leaf shedding, increased light availability in the understory, and ultimately increased abundance of the grass Deschampsia flexulosa.108 c 2010 New York Academy of Sciences. Ann. N.Y. Acad. Sci. 1195 (2010) 46–61 ! 55 Nitrogen enrichment and plant communities Cleland & Harpole Pathogens may also play a role in altered plant– soil feedbacks with N enrichment.109 For instance, N addition tends to alter microbial communities by increasing the relative abundance of fungi over bacteria,110 although dominant plant composition had a greater effect on overall microbial biomass and composition in this study. Mycorrhizal composition also changes in response to N addition,111,112 although it is not known if the facilitative/pathogenic balance in this essential plant–microbe interaction is altered. Synthesis and conclusions Synthesis and future research needs Recent empirical and conceptual advances in our understanding of resource limitation and the impacts of changing nutrient inputs to ecosystems have contributed to a better understanding of the ecology of terrestrial ecosystems, but they also highlight several areas that are not well understood. N and P colimitation appears to be common; we know relatively little about the importance of other nutrients in natural systems, in particular whether they are interactively colimiting with N and P. Greater efforts are being made to monitor nutrient deposition around the world. Such data will provide important regional and continental scale estimates of deposition trends. Smaller scale data are also needed because regional scale extrapolations can greatly underestimate local deposition rates and it is the local scale variation that is important for understanding plant community response to atmospheric deposition. Because ecological systems are enormously complex, many studies focus on single factors, yet the pattern we see is that interactions are not only common but often expected theoretically: interactions between plant species, multiple resources, trophic levels, climate change factors, etc. Our conceptual ability to interpret higher order interactions is limited, as is our data to test them. We study species that for the most part evolved in a preindustrial world and all areas of the world are impacted by some aspect of global change. Species traits reflect adaptations to environmental conditions that either no longer exist or are changing rapidly. There is likely to be a growing mismatch between evolved species traits and novel resource and limiting factor environments. We need to bet- 56 ter synthesize evolution and ecology to understand the implications for biodiversity and conservation of a world and its species, which may all be changing at different rates. Many of our recent advances in understanding have come from meta-analysis across many studies that are similar. A greater challenge will be to synthesize across different kinds of studies (e.g., observational and experimental) and identify gaps in our understanding that can only be filled by imagining new types of studies that are different than our conventional approaches. Management implications In Europe, there have been intense research efforts to set and refine “critical loads” for N deposition, defined as “a quantitative estimate of an exposure to one or more pollutants below which significant harmful effects on specified sensitive elements of the environment do not occur according to present knowledge.”113 This research was driven by demand for estimates of critical loads by international European air pollution treaties; critical loads have been an established component of European pollution policy for over 20 years and have been successful in reversing ecosystem acidification trends. Lacking an analogous legislative demand, critical loads have not been developed for the United States, although there are federal mandates to protect public lands from the effects of N deposition (reviewed by Porter et al.114 ). The recent formation of a Critical Loads sub-committee within the National Atmospheric Deposition Program within the Environmental Protection Agency suggests that there is growing interest in this concept in the United States.115 . A variety of approaches have been proposed for the definition of critical loads in the United States, including empirical approaches based on long-term ecological monitoring,116 identifying sensitive indicator species,117 “ecological hindcasting,”81 and simulation models of N saturation and subsequent nitrate leaching and acidification.47,118 It is unclear how the rate of community and ecosystem recovery will proceed once N inputs are reduced below critical loads,119 and whether systems will return to their original state or experience hysteresis, remaining in an altered state.120,121 Careful experiments are required to identify threshold responses of sensitive communities and ecosystems to N deposition,69,122 both in terms of initial c 2010 New York Academy of Sciences. Ann. N.Y. Acad. Sci. 1195 (2010) 46–61 ! Cleland & Harpole responses to enrichment, and levels below which recovery from enrichment is possible.121 Conclusion Nitrogen is an important limiting nutrient across ecosystems, but consistent and globally general patterns of colimitation highlight the importance of other resources such as phosphorus availability and precipitation inputs. We know far less about the importance of other limiting nutrients in structuring terrestrial plant communities, in particular when there are important connections between nutrient cycles, such as between N and P via N fixation. Super-abundant N (and lower pH) should lead to new scarcities and potential for release of other elements in soil that could increase toxicity; species best adapted to those novel conditions are likely to be different than those found in current communities adapted to low N environments. Numerous experimental and observational studies suggest that as N enrichment proceeds, communities become increasingly dominated by a fewer species, particularly when productivity is increased. Although competition for other resources such as water or light is frequently invoked as the mechanism to explain diversity declines in concert with increasing productivity, we are beginning to understand the importance of other biotic interactions such as herbivory or disease that could mediate these responses. As policymakers and land managers seek to limit N deposition through development of critical loads or other legislative mechanisms, ecologists have the opportunity to inform the outcome by communicating their knowledge about the importance of N in structuring natural plant communities, as well as the importance of environmental and biotic context that mediates community responses to N deposition. Acknowledgments The authors are grateful to an anonymous reviewer for detailed and constructive comments on an earlier draft of this review. The authors are also grateful to Steven Porder and Jim Dalling for constructive conversations that greatly improved particular sections of this review. Conflicts of interest The authors declare no conflicts of interest. Nitrogen enrichment and plant communities References 1. Vitousek, P.M. & R.W. Howarth. 1991. Nitrogen limitation on land and in the sea – how can it occur? Biogeochemistry 13: 87–115. 2. Smil, V. The Earth as Transformed by Human Action. 1990. B.L. Turner et al., Eds.: 423–436. Cambridge University Press. Cambridge. 3. Galloway, J. & E. Cowling. 2002. Reactive nitrogen and the world: 200 years of change. Ambio 31: 64–71. 4. Gruber, N. & J. N. Galloway. 2008. An Earth-system perspective of the global nitrogen cycle. Nature 45: 293– 296. 5. Galloway, J.N. et al. 2004. Nitrogen cycles: past, present, and future. Biogeochemistry 70: 153–226. 6. Vitousek, P. et al. 1997. Human alteration of the global nitrogen cycle: sources and consequences. Ecol. Appl. 7: 737–750. 7. DiTommaso, A. & L. W. Aarssen. 1989. Resource manipulations in natural vegetations: a review. Vegetation 84: 9–29. 8. Bobbink, R., M. Hornung & J. G. M. Roelofs. 1998. The effects of air-borne nitrogen pollutants on species diversity in natural and semi-natural European vegetation. J. Ecol. 86: 717–738. 9. Cleveland, C.C. et al. 1999. Global patterns of terrestrial biological nitrogen (N-2) fixation in natural ecosystems. Global Biogeochem. Cycles 13: 623–645. 10. Lelieveld, J. & F. J. Dentener. 2000. What controls tropospheric ozone? J. Geophys. Res.-Atmos. 105(D3): 3531– 3551. 11. Chapin, F.S. 1980. The mineral-nutrition of wild plants. Annu. Rev. Ecol. Syst. 11: 233–260. 12. Schimel, J. P. & J. Bennett. 2004. Nitrogen mineralization: challenges of a changing paradigm. Ecology 85: 591–602. 13. McKane, R.B. et al. 2002. Resource-based niches provide a basis for plant species diversity and dominance in arctic tundra. Nature 415: 68–71. 14. Matson, P.A., W.H. McDowell, A.R. Townsend & P.M. Vitousek. 1999. The globalization of N deposition: ecosystem consequences in tropical environments. Biogeochemistry 46: 67–83. 15. Vitousek, P.M., S. Porder, B.Z. Houlton & O.A. Chadwick. 2010. Terrestrial phosphorus limitation: mechanisms, implications, and nitrogen-phosphorus interactions. Ecol. Appl. 20: 5–15. 16. Falkowski, P. et al. 2000. The global carbon cycle: a test of our knowledge of Earth as a system. Science 290: 291–294. c 2010 New York Academy of Sciences. Ann. N.Y. Acad. Sci. 1195 (2010) 46–61 ! 57 Nitrogen enrichment and plant communities Cleland & Harpole 17. Newman, E.I. 1995. Phosphorus inputs to terrestrial ecosystems. J. Ecol. 83: 713–726. 18. Chapin F.S. III, P.A. Matson & H.A. Mooney. 2002. Principles of Terrestrial Ecosystem Ecology. Springer. New York. 19. Knops, J.M.H. & D. Tilman. 2000. Dynamics of soil nitrogen and carbon accumulation for 61 years after agricultural abandonment. Ecology (Washington, DC) 81: 88–98. 20. Walker, T.W. & J.K. Syers. 1976. The fate of phosphorus during pedogenesis. Geoderma 15: 1–19. 21. Vitousek, P.M. & H. Farrington. 1997. Nutrient limitation and soil development: experimental test of a biogeochemical theory. Biogeochemistry 37: 63–75. 22. Raich, J.W., A.E. Russell, T.E. Crews et al. 1996. Both nitrogen and phosphorus limit plant production on young Hawaiian lava flows. Biogeochemistry 32: 1–14. 23. Sterner, R.W. & J.J. Elser. 2002. Ecological Stoichiometry: The Biology of Elements from Molecules to the Biosphere. Princeton University Press. Princeton. 24. Elser, J.J. et al. 2003. Growth rate-stoichiometry couplings in diverse biota. Ecol. Lett. 6: 936–943. 25. Van Mooy, B.A. et al. 2009. Phytoplankton in the ocean use non-phosphorus lipids in response to phosphorus scarcity. Nature 458: 69–72. 26. Tilman, D. 1982. Resource Competition and Community Structure. Princeton University Press. Princeton. 27. Harpole, W.S. & D. Tilman. 2007. Grassland species loss due to reduced niche dimension. Nature 446: 791–793. 28. Hautier, Y., P.A. Niklaus & A. Hector. 2009. Competition for light causes plant biodiversity loss after eutrophication. Science 324: 636–638. 29. Reynolds, H.L., G.G. Mittelbach, T.L. Darcy-Hall et al. 2007. No effect of varying soil resource heterogeneity on plant species richness in a low fertility grassland. J. Ecol. 95: 723–733. 30. Bloom, A.J., F.S. Chapin & H.A. Mooney. 1985. Resource limitation in plants: an economic analogy. Annu. Rev. Ecol. Syst. 16: 363–392. 31. Rastetter, E.B. & G.R. Shaver. 1992. A model of multiple-element limitation for acclimating vegetation. Ecology 73: 1157–1174. 32. Gleeson, S.K. & D. Tilman. 1992. Plant allocation and the multiple limitation hypothesis. Am. Nat. 139: 1322– 1343. 33. Vance, C.P., C. Uhde-Stone & D.L. Allan. 2003. Phosphorus acquisition and use: critical adaptations by plants for securing a nonrenewable resource. New Phytol. 157: 423–447. 34. Chapman, S.K., J.A. Langley, S.C. Hart & G.W. Koch. 58 35. 36. 37. 38. 39. 40. 41. 42. 43. 44. 45. 46. 47. 48. 49. 2006. Plants actively control nitrogen cycling: uncorking the microbial bottleneck. New Phytol. 169: 27– 34. Wedin, D.A. & D. Tilman. 1990. Species effect on nitrogen cycle: a test with perennial grasses. Oecologia 84: 433–441. Knops, J.M.H., K.L. Bradley & D.A. Wedin. 2002. Mechanisms of plant species impacts on ecosystem nitrogen cycling. Ecol. Lett. 5: 454–466. Danger, M., T. Daufresne, F. Lucas et al. 2008. Does Liebig’s law of the minimum scale up from species to communities? Oikos 117: 1741–1751. Wedin, D. & D. Tilman. 1993. Competition among grasses along a nitrogen gradient: initial conditions and mechanisms of competition. Ecol. Monogr. 63: 199–229. Tilman, D. 1987. The importance of mechanisms of interspecific competition. Am. Nat. 129: 769–774. Weiher, E., G.D.P. Clarke & P.A. Keddy. 1998. Community assembly rules, morphological dispersion, and the coexistence of plant species. Oikos 81: 309–322. Chapin F.C. III, P.M. Vitousek & K. V. Cleve. 1986. The nature of nutrient limitation in plant communities. Am. Nat. 127: 48–58. Craine, J.M. et al. 2002. Functional traits, productivity and effects on nitrogen cycling of 33 grassland species. Funct. Ecol. 16: 563–574. Harpole, W.S. & D. Tilman. 2006. Non-neutral patterns of species abundance in grassland communities. Ecol. Lett. 9: 15–23. Howarth, R.W., E.W. Boyer, W.J. Pabich & J.N. Galloway. 2002. Nitrogen use in the United States from 1961–2000 and potential future trends. Ambio 31: 88– 96. Vitousek, P.M. et al. 1997. Human alteration of the global nitrogen cycle: sources and consequences. Ecol. Appl. 7: 737–750. Holland, E.A., B.H. Braswell, J. Sulzman & J.F. Lamarque. 2005. Nitrogen deposition onto the United States and western Europe: synthesis of observations and models. Ecol. Appl. 15: 38–57. Schulze, E.D., W. d. Vries & M. Hauhs. 1989. Critical loads for nitrogen deposition on forest ecosystems. Water, Air and Soil Pollution 48: 451–456. Aber, J. et al. 1998. Nitrogen saturation in temperate forest ecosystems – hypotheses revisited. Bioscience 48: 921–934. Roem, W.J., H. Klees & F. Berendse. 2002. Effects of nutrient addition and acidification on plant species diversity and seed germination in heathland. J. Appl. Ecol. 39: 937–948. c 2010 New York Academy of Sciences. Ann. N.Y. Acad. Sci. 1195 (2010) 46–61 ! Cleland & Harpole 50. Matson, P.A., K.A. Lohse & S.J. Hall. 2002. The globalization of nitrogen deposition: consequences for terrestrial ecosystems. Ambio 31: 113–119. 51. Galloway, J.N. et al. 2003. The nitrogen cascade. Bioscience 53: 341–356. 52. Stevens, C.J., N.B. Dise, J.O. Mountford & D.J. Gowing. 2004. Impact of nitrogen deposition on the species richness of grasslands. Science 303: 1876–1879. 53. Morris, J.T. 1991. Effects of nitrogen loading on wetland ecosystems with particular reference to atmospheric deposition. Annu. Rev. Ecol. Syst. 22: 257–279. 54. Bedford, B.L., M.R. Walbridge & A. Aldous. 1999. Patterns in nutrient availability and plant diversity of temperate North American wetlands. Ecology 80: 2151– 2169. 55. Fenn, M.E. et al. 2003. Ecological effects of nitrogen deposition in the western United States. Bioscience 53: 404–420. 56. Phoenix, G.K. et al. 2006. Atmospheric nitrogen deposition in world biodiversity hotspots: the need for a greater global perspective in assessing N deposition impacts. Global Change Biol. 12: 470–476. 57. Gough, L., C. Osenberg, K. Gross & S. Collins. 2000. Fertilization effects on species density and primary productivity in herbaceous plant communities. Oikos 89: 428–439. 58. Suding, K.N. et al. 2005. Functional- and abundancebased mechansisms explain diversity loss due to N fertilization. Proc. Natl. Acad. Sci. 102: 4387– 4392. 59. Hillebrand, H. et al. 2007. Consumer versus resource control of producer diversity depends on ecosystem type and producer community structure. Proc. Natl. Acad. Sci. 104: 10904–10909. 60. Collins, S.L. et al. 2008. Rank clocks and community dynamics. Ecology 89: 3534–3541. 61. Lamb, E.G. 2008. Direct and indirect control of grassland community structure by litter, resources, and biomass. Ecology 89: 216–225. 62. Xia, J. & S. Wan. 2008. Global response patterns of terrestrial plant species to nitrogen addition. New Phytol. 179: 428–439. 63. Daehler, C.C. 2003. Performance comparisons of cooccurring native and alien invasive plants: implications for conservation and restoration. Annu. Rev. Ecol. Evol. Syst. 34: 183–211. 64. Stevens, C.J., N.B. Dise, D.J.G. Gowing & J.O. Mountford. 2006. Loss of forb diversity in relation to nitrogen deposition in the UK: regional trends and potential controls. Global Change Biol. 12: 1823–1833. Nitrogen enrichment and plant communities 65. Zavaleta, E. et al. 2003. Grassland responses to three years of elevated temperature, CO2, precipitation, and N deposition. Ecol. Monogr. 73: 585–604. 66. Huenneke, L.F., S.P. Hamburg, R. Koide, et al. 1990. Effects of soil resources on plant invasion and community structure in Californian serpentine grassland. Ecology 71: 478–491. 67. Oksanen, J. 1996. Is the humped relationship between species richness and biomass an artefact due to plot size? J. Ecol. 84: 293–295. 68. Chalcraft, D.R. et al. 2008. Scale-dependent responses of plant biodiversity to nitrogen enrichment. Ecology 89: 2165–2171. 69. Clark, C. M. & D. Tilman. 2008. Loss of plant species after chronic low-level nitrogen deposition to prairie grasslands. Nature 451: 712–715. 70. Brenchley, W. E. & K. Warington. 1958. The park grass plots at rothamsted 1856–1949. The Park Grass plots at Rothamsted 1856–1949. 71. Crawley, M.J. et al. 2005. Determinants of species richness in the Park Grass Experiment. Am. Nat. 165: 179– 192. 72. Tilman, D. et al. 1994. Long-Term Experiments in Agricultural and Ecological Sciences R. A. Leigh & A. E. Johnston, Eds.: CAB International. Wallingford. 73. Silvertown, J., P.M. Biss & J. Freeland. 2009. Community genetics: resource addition has opposing effects on genetic and species diversity in a 150-year experiment. Ecol. Lett. 12: 165–170. 74. Elser, J.J. et al. 2007. Global analysis of nitrogen and phosphorus limitation of primary producers in freshwater, marine, and terrestrial ecosystems. Ecol. Lett. 10: 1135–1142. 75. Hutchinson, G.E. 1957. Concluding remarks. Cold spring harbor symposium. Quant. Biol. 22: 415–427. 76. Olde Venterink, H., M.J. Wassen, A.W.M. Verkroost & P.C. de Ruiter. 2003. Species richness-productivity patterns differ between N-, P-, and K-limited wetlands. Ecology 84: 2191–2199. 77. Braakhekke, W.G. & D.A.P. Hooftman. 1999. The resource balance hypothesis of plant species diversity in grassland. J. Veg. Sci. 10: 187–200. 78. Herbert, D.A., E.B. Rastetter, L. Gough & G.R. Shaver. 2004. Species diversity across nutrient gradients: an analysis of resource competition in model ecosystems. Ecosystems 7: 296–310. 79. Cardinale, B.J., H. Hillebrand, W.S. Harpole et al. 2009. Separating the influence of resource ‘availability’ from resource ‘imbalance’ on productivity–diversity relationships. Ecol. Lett. 12: 475–487. c 2010 New York Academy of Sciences. Ann. N.Y. Acad. Sci. 1195 (2010) 46–61 ! 59 Nitrogen enrichment and plant communities Cleland & Harpole 80. Richter, A., J.P. Burrows, H. Nüss et al. 2005. Increase in tropospheric nitrogen dioxide over China observed from space. Nature 437: 129–132. 81. Baron, J.S. 2006. Hindcasting nitrogen deposition to determine an ecological critical load. Ecol. Appl. 16: 433–439. 82. Pennings, S.C. et al. 2005. Do individual plant species show predictable responses to nitrogen addition across multiple experiments? Oikos 110: 547–555. 83. Clark, C.M. et al. 2007. Environmental and plant community determinants of species loss following nitrogen enrichment. Ecol. Lett. 10: 596–607. 84. Churkina, G. & S.W. Running. 1998. Contrasting climatic controls on the sstimated productivity of global terrestrial biomes. Ecosystems 1: 206–215. 85. Harpole, W.S., D.L. Potts & K.N. Suding. 2007. Ecosystem responses to water and nitrogen amendment in a California grassland. Global Change Biol. 13: 1–8. 86. Field, C. & H.A. Mooney. 1986. On the Economy of Plant form and Function. T. Givnish 25–55. Cambridge University Press. Cambridge. 87. Fenn, M.E. et al. 2003. Nitrogen emissions, deposition, and monitoring in the western United States. Bioscience 53: 391–403. 88. Báez, S., J. Fargione, D.I. Moore et al. 2006. Atmospheric nitrogen deposition in the northern Chihuahuan desert: temporal trends and potential consequences. J. Arid Environ. 68: 640–651. 89. Mitchell, M.J. et al. 1996. Climatic control of nitrate loss from forested watersheds in the northeast United States. Environ. Sci. Technol. 30: 2609–2612. 90. Hooper, D.U. & L. Johnson. 1999. Nitrogen limitation in dryland ecoystems: responses to geographical and temporal variation in precipitation. Biogeochemistry 46: 247–293. 91. Goldberg, D.E. & T. Miller. 1990. Effects of different resource additions on species diversity in an annual plant community. Ecology 71: 213–225. 92. Tilman, D. 1988. Plant Strategies and the Dynamics and Structure of Plant Communities, Vol. 26. Princeton University Press. Princeton. 93. Foster, B.L. & K.L. Gross. 1998. Species richness in a successional grassland: effects of nitrogen enrichment and plant litter. Ecology 79: 2593–2602. 94. Rajaniemi, T.K. 2002. Why does fertilization reduce plant species diversity? Testing three competition-based hypotheses. J. Ecol. 90: 316–324. 95. Gruner, D.S. et al. 2008. A cross-system synthesis of consumer and nutrient resource control on producer biomass. Ecol. Lett. 11: 740–755. 60 96. Throop, H.L. & M.T. Lerdau. 2004. Effects of nitrogen deposition on insect herbivory: implications for community and ecosystem processes. Ecosystems 7: 109– 133. 97. Mattson, W.J. 1980. Herbivory in relation to plant nitrogen content. Annu. Rev. Ecol. Syst. 11: 119–161. 98. Rosenzweig, M.L. 1971. Paradox of enrichment: destabilization of exploitation ecosystems in ecological time. Science 171: 385–387. 99. Hillebrand, H. et al. 2009. Herbivore metabolism and stoichiometry each constrain herbivory at different organizational scales across ecosystems. Ecol. Lett. 12: 516–527. 100. Jones, M.B. et al. 1990. Effects of phosphorus and sulfur fertilization on subclover-grass pasture production as measured by lamb gain. J. Prod. Agric. 3: 534– 539. 101. Mitchell, C.E. 2003. Trophic control of grassland production and biomass by pathogens. Ecol. Lett. 6: 147– 155. 102. Gilbert, G.S. 2002. Evolutionary ecology of plant diseases in natural ecosystems. Annu. Rev. Phytopathol. 40: 13–43. 103. Strengbom, J., A. Nordin, T. Nasholm & L. Ericson. 2002. Parasitic fungus mediates change in nitrogenexposed boreal forest vegetation. J. Ecol. 90: 61–67. 104. Nordin, A., T. Näsholm & L. Ericson. 1998. Effects of simulated N deposition on understorey vegetation of a boreal coniferous forest. Funct. Ecol. 12: 691–699. 105. Paul, N.D. 1990. Pests, Pathogens and Plant Communities. J.J. Burdon & S.R. Leather, Eds.: 81–96. Blackwell. Oxford. 106. Strengbom, J. & P.B. Reich. 2006. Elevated [CO2] and increased N supply reduce leaf disease and related photosynthetic impacts on Solidago rigida. Oecologia 149: 519–525. 107. Hoffland, E., M.J. Jeger & M.L. van Beusichem. 2000. Effect of nitrogen supply rate on disease resistance in tomato depends on the pathogen. Plant Soil 210: 239– 247. 108. Strengbom, J., T. Näsholm & L. Ericson. 2004. Light, not nitrogen, limits growth of the grass Deschampsia flexuosa in boreal forests. Can. J. Bot. 82: 430–435. 109. Manning, P., S.A. Morrison, M. Bonkowski & R.D. Bardgett. 2008. Nitrogen enrichment modifies plant community structure via changes to plant–soil feedback. Oecologia 157: 661–673. 110. Bardgett, R.D. et al. 1999. Plant species and nitrogen effects on soil biological properties of temperate upland grasslands. Funct. Ecol. 13: 650–660. c 2010 New York Academy of Sciences. Ann. N.Y. Acad. Sci. 1195 (2010) 46–61 ! Cleland & Harpole 111. Treseder, K.K. 2004. A meta-analysis of mycorrhizal responses to nitrogen, phosphorus, and atmospheric CO2 in field studies. New Phytol. 164: 347– 355. 112. Johnson, N.C., D.L. Rowland, L. Corkidi et al. 2003. Nitrogen enrichment alters mycorrhizal allocation at five mesic to semiarid grasslands. Ecology 84: 1895– 1908. 113. Nilsson, J. & P. Grennfelt. 1988. Critical loads for sulfur and nitrogen. Report for the Nordic Council of Ministers, Copenhagen, Denmark. 114. Porter, E., T. Blett, D.U. Potter & C. Huber. 2005. Protecting resources on federal lands: implications of critical loads for atmospheric deposition of nitrogen and sulfur. Bioscience 55: 603–612. 115. Burns, D.A., T. Blett, R. Haeuber & L.H. Pardo. 2008. Critical loads as a policy tool for protecting ecosystems from the effects of air pollutants. Front. Ecol. Env. 6: 156–159. 116. Williams, M.W. & K.A. Tonnessen. 2000. Critical loads for inorganic nitrogen deposition in the Colorado Front Range, USA. Ecol. Appl. 10: 1648–1665. 117. Bowman, W.D., J.L. Gartner, K. Holland & M. Wiedermann. 2006. Nitrogen critical loads for alpine Nitrogen enrichment and plant communities 118. 119. 120. 121. 122. 123. c 2010 New York Academy of Sciences. Ann. N.Y. Acad. Sci. 1195 (2010) 46–61 ! vegetation and terrestrial ecosystem response—are we there yet? Ecol. Appl. 16: 1183–1193. Driscoll, C.T. et al. 2003. Nitrogen pollution in the northeastern United States: sources, effects, and management options. Bioscience 53: 357–374. Nordin, A., J. Strengbom, J. Witzell et al. 2005. Nitrogen deposition and the biodiversity of boreal forests: implications for the nitrogen critical load. Ambio 34: 20–24. Folke, C. et al. 2004. Regime shifts, resilience, and biodiversity in ecosystem management. Annu. Rev. Ecol., Evol. Syst. 35: 557–581. Clark, C.M., S.E. Hobbie, R. Venterea & D. Tilman. 2009. Long-lasting effects on nitrogen cycling 12 years after treatments cease despite minimal longterm nitrogen retention. Global Change Biol. 15: 1755– 1766. Groffman, P. et al. 2006. Ecological thresholds: the key to successful environmental management or an important concept with no practical application? Ecosystems 9: 1–13. Monteith, D.T. et al. 2007. Dissolved organic carbon trends resulting from changes in atmospheric deposition chemistry. Nature 450: 537–540. 61