* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download Neuroprotection in Dementia
Survey
Document related concepts
Transcript
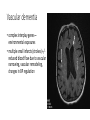
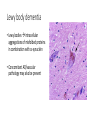
Neuroprotection: Strategies for Dementia Sesath Hewapathirane PGY3 Resident in Psychiatry (UBC) May 25, 2017 Dementia: clinical definition • decline in cognition involving one or more of the following cognitive domains: • • • • • • learning and memory: short and long-term memory language: expressive and receptive executive function: complex tasks, overall insight + judgement complex attention: sustained/divided/selective attention, processing speed perceptual-motor: apraxia, agnosia, perceptual distortions, visuospatial orientation social cognition: personality changes • decline from previous level of function • interfere with daily function and independence • progressive illness, eventually unable to care for themselves Dementia: prevalence and burden • 47.5 million affected worldwide • mainly seen in people over age 65 (early onset variants also exist) • prevalence increases steeply with age • starting age 65, risk doubles every 5 years • by age 85, 25-50% will exhibit signs of dementia • 3rd leading cause of death in developed world (after cadiovasc. and cancer) • significant illness burden (US$604 billion/yr) • hospitalizations, specialized medical facilities + staff + treatments • home supports, care facilities • societal burden (caregivers’ mental health and income potential) • prevalence on the rise (longer life expectancy +/- exposures) Dementia: subtypes • over 50 different causes • • • • Alzheimer’s disease: 50%-70% Vascular dementia: 20%-30% Lewy body dementia: 10-15% Fronto-temporal dementia • • • • • Traumatic brain injury Inherited e.g. Huntington’s Infectious e.g. HIV, prion Substance-related e.g. alcohol Other neurological/medical conditions e.g. Parkinson’s disease • mixed etiologies also observed • clinical characteristics vary based on brain areas affected, may also demonstrate different time-courses Alzheimer’s dementia • cause is poorly understood • genetic (multiple genes, est 100-1000s) • environmental (e.g. head injuries, depression, hypertension) • toxic plaques and tangles in the brain, temporal lobes often affected Alzheimer’s dementia • Neuritic plaques and neurofibrillary tangles neuron death • Amyloid hypothesis – abnormal cleavage of APP to β-amyloid peptide (Aβ)/N-APP/other extracellular amyloid plaques • Tau hypothesis – hyperphosphorylation of Tau protein leading to microtubule disintegration and cytoskeletal collapse intracellular NFTs Amyloid hypothesis • Positive feedback prionic loops • APP as a mediator of synaptic plasticity signaling • Endogenous molecular switch • Anti-tropic peptides shown to mediate neurite retraction, loss of synapses, caspase activation, apoptosis • AD a result of imbalance of APP singaling • anti-trophic>>tropic Vascular dementia • complex interplay genes— environmental exposures • multiple small infarcts (strokes) +/reduced blood flow due to vascular narrowing, vascular remodeling, changes in BP regulation Lewy body dementia • Lewy bodies intracellular aggregations of misfolded proteins in combination with α-synuclein • Concomitant AD/vascular pathology may also be present Dementia: treatment • few available medications aim to treat cognitive symptoms but have no effect on the underlying disease process • cholinesterase inhibitors: donepezil, rivastigmine*, galantamine (mildmoderate AD and *PDD) • NMDAR antagonists: memantine (moderate – severe AD) • clinical benefits often minimal • benefits are transient (6-24 months) Disease modifying treatments for AD Aβ therapeutics • Immunization with Aβ • trial halted in phase II due to meningoencephalitis (6%), presumed T-cell hyperresponse • post-mortem, fewer plaques but did not prevent clinical progression to severe dementia • ongoing trials with truncated Aβ (aimed at circumventing T-cell hyperresponse) completed phase II without reports of encephalitis • Passive immunization with pre-formed antibodies against Aβ • Several large scale phase III studies, primarily negative results • Bapineuzumab observed to reduce amyloid levels (PET) and CSF phos-Tau however failed to show improvement in clinical outcomes • Solanezumab showed mixed results with one meta-analysis of 2 studies indicate very mild benefit in cognition in people with mild AD • Was therapy initiated too late in the disease process? • Crenezumab, solanezumab, gantenerumab in “prodromal” AD & familial AD APP cleavage • Gamma-secretase inhibitors, (e.g. avagacestat, semagacestat) • no improvement in cognitive function reported in Phase II trials • trend towards a dose-dependent decrease in cognition • more APP-specific inhibitors in pipeline? • Beta-secretase inhibitor (MK-8931) • Phase III ongoing, animal studies shown sig. side effects… Anti-Tau therapies • Target tau-tau binding methylene blue-based compounds • Rember (Phase II) reduced cognitive decline over 24 weeks • TRx0237 (Phase III) ongoing • Target tau-phosphorylation Glycogen synthase-kinase 3β • sodium valproate showed no benefit in Phase III trials • Li showed stabilization of cognitive function with low dose Tx, delayed d/t adverse Tx effects • tideglusib failed Phase II trials Other directions in early stages • α-secretase upregulators (etazolate, bryostatin-1, exebryl-1) • Microtubule stabilizers • Repression of AD-related transcription factors AD Tx: Drug repurposing • Pro: existing safety data and established mechanisms of action in order to bring to clinical trial rapidly • Type 2 diabetes mellitus Tx: • 65% increased risk of developing AD in T2DM • Hypothesized that disrupted insulin signalling contributes to AD • Phase II trial with intranasal insulin reported positive impacts on key AD markers, now progressed to Phase III • A number of Phase II and III trials are currently examining GLP-1 analogues AD Tx: Drug repurposing • Calcium channel blockers: • preclinical studies: reduction of Aβ production, aggregation and neurotoxicity and improved neuronal function • underlying mechanism is independent of the anti-hypertensive action due to the differential effects seen with the different CCBs • evidence from epidemiological studies support the efficacy of dihydropyridine CCBs in reducing or delaying the development of AD, supported by recent SYST-EUR RCT, which reported a 55% reduction in incident dementia with nitrendipine Tx over a 5-yr follow-up • Large scale RCT investigating nivaldipine is ongoing AD Tx: Drug repurposing • Trials ongoing: • ARBs • centrally acting angiotensin II has role in release of inflammatory mediators and inhibition of acetylcholine release • epidemiological evidence, mixed data from large scale RCTs ONTARGET & TRANSEND • RCTs of peridopril and losartan ongoing • Anti-inflammatories • inhibitor of mast cell activation (masitinib) shown some promise in slowing cog. decline • NSAIDs and COX-2 inhibitors have not performed well • RAGE-receptor blockers (multi-ligand receptor binding Aβ promoting influx to CNS) • Minocycline • Retinoids Dementia: the problem • • • • • >47.5 million affected (~1 in 4-5 over age 85) 3rd leading cause of death in wealthy countries >US$640 billion yearly costs immeasurable effects on family/caregivers immense burden on health care system AND: • available therapeutic interventions offer a marginal, unsustained symptomatic effect, with little or no effect on disease progression • hundreds of clinical trials, billions of dollars, minimal success • companies are teaming up – unprecedented in history of drug development Background • Hypothesized model in which AD results from an imbalance in endogenous plasticity signaling • synapse loss >> synaptogenesis & maintenance of existing connections • dysfunctional circuitry loss of brain function • preclinical studies have identified multiple pathogenic targets for potential intervention • • • • • amyloid-β (Aβ) oligomers and tau inflammatory mediators apolipoproteins and lipid metabolism factors hormonal mediators trophic factors and their receptors • • • • • calcium regulatory pathways axoplasmic transport machinery neurotransmitters and their receptors prion protein and a host of other potential targets Background • preclinical studies show large effects of targeting one pathway, whereas in human studies, such approaches have not borne out • successes with other chronic illnesses such as cardiovascular disease, neoplasia, and HIV support the efficacy of multi-component systems • Present study (n=10; 2.5 yr follow-up): • • • • novel programmatic approach involving metabolic enhancement aimed at altering balance of synapse loss & synaptogenesis/maintenance individualized, combination therapies based on research evidence iterative—continued optimization over time Multimodal approach Case • 69-year-old male, entrepreneur, with an 11 year Hx of slowly progressive memory loss with prosopagnosia, which had accelerated over the past 1-2 years. • Unable to work due to memory impairment. • FDG-PET showed a pattern typical for early Alzheimer’s disease, with reduced glucose utilization in the parietotemporal cortices bilaterally. • Neuropsychological testing showed a reduction in California Verbal Learning Test) from 84%ile to 1%ile, a Stroop color test at 16%ile, and auditory delayed memory at 13%ile. • Heterozygous for ApoE4 (3/4). Case • Underwent baseline investigations and initiated on a personalized therapeutic program: • optimize metabolic parameters • optimize lifestyle factors (exercise, sleep, stress reduction, healthy balanced diet) Case • (1) fasted for a minimum of three hours between meals, and for a minimum of 12 hours between dinner and breakfast; (2) eliminated simple carbohydrates and processed foods from his diet; (3) increased consumption of vegetables and fruits, and limited consumption of fish to non-farmed, and meat to occasional grass-fed beef or organic chicken; (4) probiotics; (5) coconut oil i tsp bid; (6) exercised strenuously, swimming 3-4 times per week, cycling twice per week, and running once per week; (7) melatonin 0.5mg po qhs, and tried to sleep as close to 8 hours per night as his schedule would allow; (8) herbs Bacopa monniera 250mg, Ashwagandha 500mg, and turmeric 400mg each day; (9) methylcobalamin 1mg, methyltetrahydrofolate 0.8mg, and pyridoxine-5-phosphate 50mg each day; (10) citicoline 500mg po bid; (11) vitamin C 1g per day, vitamin D3 5000IU per day, vitamin E 400IU per day,CoQ10 200mg per day, Zn picolinate 50mg per day, and α-lipoic acid 100mg per day; (12) he took DHA (docosahexaenoic acid) 320mg and EPA (eicosapentaenoic acid) 180mg per day. Case • Six months later, Pt and wife noted improvement. Returned to work. • At work, unlike before, able to recognize faces, remember his daily schedule, and functioned without difficulty. • Noted to be quicker with his responses. His life-long ability to add columns of numbers rapidly in his head, which he had lost during his progressive cognitive decline, returned. • Had been accelerating in his decline over the prior 1-2 years, decline appeared to have halted Author’s conclusions • memory loss in patients with subjective cognitive impairment, mild cognitive impairment, and at least the early phase of AD may be reversed • subjective improvement sustained for 2.5 years • 6 of the 10 patients had discontinued working; all were able to return to work • major side effect – improved health and optimal BMI; stark contrast to medications • may be useful as a platform on which drugs that would fail as monotherapeutics may succeed as key components of a multimodal therapeutic system • program is not easy; none of the patients followed the entire protocol • the current, anecdotal results require a larger trial Design • n=10, on MEND protocol for 5-24 months, up to 4 years follow-up • quantitative MRI and neuropsychological testing pre/post • heterogeneous measures not amenable for statistical analysis Examples of outcomes • well documented MCI, positive amyloid-PET and FDG-PET scans, abnormal neuropsychological testing, hippocampal volume reduced to 17th percentile; after 10 months on the MEND protocol, hippocampal volume increased to 75th percentile, in association with a reversal of cognitive decline. • well documented early Alzheimer’s disease, positive FDG-PET scan and markedly abnormal neuropsychological testing. After 22 months on the MEND protocol, CVLT-IIB had increased from 3rd percentile to 84th percentile, total recognized hits from <1st percentile to 50th percentile, CVLT-II from 54th percentile to 96th percentile, auditory delayed memory from 13th percentile to 79th percentile, reverse digit span from 24th percentile to 74th percentile, and processing speed from 93rd percentile to 98th percentile. His business, which had been in the process of termination, was reinvigorated, and a new site was added to the previous sites of operation. Limitations + Future directions • pilot/anecdotal • cannot rule out reversible non-AD etiologies in the subject group (?psychiatric, ?improved perfusion) • most SCI/MCI or early AD; how late in the course of cognitive decline can reversal be effected? • MCI does not necessarily progress to dementia • how long can improvement be sustained? • compliance, cost • larger study size, +/- existing medications • curative therapies in pipeline Paradigm shift in Tx of MCI/Dementia • • • • • >47.5 million affected (~1 in 4-5 over age 85) no current disease modifying treatments immense personal/family/societal burden unsustainably growing costs to healthcare system (very) early clinical data exists showing potential to reverse cognitive decline in MCI/early AD • Utilization of personalized, assertive, iterative programs to optimize potential risk factors; multimodal approach targeting several pathways promoting neuroprotection • Consider as primary focus of treatment plan, versus “supportive”