* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download genetics by jude hayward
Human genetic variation wikipedia , lookup
Quantitative trait locus wikipedia , lookup
Heritability of IQ wikipedia , lookup
History of genetic engineering wikipedia , lookup
Genetic engineering wikipedia , lookup
Designer baby wikipedia , lookup
Fetal origins hypothesis wikipedia , lookup
Pharmacogenomics wikipedia , lookup
Oncogenomics wikipedia , lookup
Population genetics wikipedia , lookup
Behavioural genetics wikipedia , lookup
Microevolution wikipedia , lookup
BRCA mutation wikipedia , lookup
Nutriepigenomics wikipedia , lookup
Genetic testing wikipedia , lookup
Genome (book) wikipedia , lookup
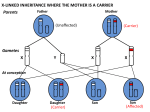
Genetics in Primary Care Dr. Jude Hayward GPwSI in Genetics, YRGS Dr. Brooke & Ptrs, Bradford. The Genetics Explosion: ‘Cancer: 'How I beat my DNA timebomb’’ ‘'Designer baby' to be free from breast cancer: A British woman has made history by conceiving the country's first "designer baby" guaranteed to be free from hereditary breast cancer.’ ‘GM food needed to avert global crisis, says Government adviser’ ‘New DNA profiling technology could tell police who suspects are in under an hour: QuickTime™ and a TIFF (Uncompressed) decompressor are needed to see this picture. A new portable device that can identify suspects in less than an hour using DNA left at the scene of a crime is set to transform the way police track down criminals.’ What does Genetics mean to you? Tricky Dry Highly Specialised – sometimes the patients know more than you do Interesting challenge Difficult to explain to patients The murky quagmire you don’t want to enter…. What does ‘genetics’ mean to you? Craniofaciocutaneous Syndrome Mental retardation ASD / HOCM Icthyosis Sparse Hair High Forehead Prominent ears Depressed nasal bridge Family History – why do we do it? Think of the patient you most recently asked for a family history – what was the situation / presenting problem? What did you do with that information? Family History – why do we do it? To aid with accurate risk assessment - likelihood of developing a certain disease To identify those who have an underlying genetic condition who would benefit from further information and services To identify other members of the family who may be at risk This leads to appropriate management strategies Role of Primary Health Care Team (RCGP) General Practitioners have a key role in identifying patients and families who would benefit from being referred to appropriate specialist genetic services Management and support of families with / at risk of genetic conditions Consideration of FH in multi-factorial disease e.g. cancer, DM, CHD Communication of Genetic Information What would you like to know? Objectives for today’s session Through discussion of cases: To outline the scope of genetic issues in primary care To discuss some common presentations of patients with genetic issues To identify some useful guidelines and resources for clinicians To identify useful resources for patients To touch on basic genetics / inheritance patterns To outline the structure of services providing care to patients with genetic issues To encourage ‘thinking Genetics’ where you might not have done before! Scope of Genetics in Primary Care 10% of consultations have genetic aspect Mostly multifactorial disease with genetic component e.g. CHD, asthma, Alzheimer's, diabetes Single gene disorders e.g. CF, Huntingdon’s, Reproductive issues e.g. Hbopathies (Genomic Medicine) (Pharmacogenetics) Multifactorial Inheritance Increased risk on basis of Family History Environmental factors ‘nurture’ Autosomally inherited / Single/gene Condition Genetic Factors ‘nature’ A typical morning surgery… A ‘typical’ morning surgery… Mrs. B, aged 52, attends for a blood pressure check as she has had 2 raised readings over the last few months. Today it is 152 / 96. She says she’s not surprised it is raised as she has just heard that her sister has been diagnosed with ovarian cancer – this has come as a shock as she has been supporting her other sister through a course of chemotherapy for breast cancer. What else would you ask? Cancer is common 1 in 3-4 Of the general population will develop cancer during their lifetime Breast cancer: 1 in 8 women Ovarian cancer: 1 in 50 women Bowel cancer: 1 in 18 men, 1 in 20 women Incidence increases with age (risk factor) Multifactorial Inheritance Increased risk on basis of Family History Environmental factors ‘nurture’ Autosomally inherited / Single/gene Condition Genetic Factors ‘nature’ Hereditary Cancer Syndromes 1 in 20 cases of breast, ovarian, CRC cancer are the result of an underlying hereditary cancer syndrome. Breast/ovarian cancer syndromes: BRCA 1 + 2 Breast (80% lifetime risk), Ovarian (40% lifetime risk) Ass. cancers: Male Breast Cancer, Prostate Cancer, Certain melanomas, association with CML / renal cell carcinoma Colorectal cancer syndromes: FAP / HNPCC CRC: FAP (100% lifetime risk) HNPCC (80% risk in males) HNPCC associated cancers: ovarian, endometrial, gastric, biliary, urinary tract Autosomal Dominant Inheritance Family history… Who affected (maternal and paternal sides) How many relatives affected Cancer type Age at diagnosis Other risk factors (e.g. smoking) She tells you that her: Sister: breast cancer aged 52 Sister: ovarian cancer aged 48 Father’s sister: breast cancer aged 61 Risk assessment Familial Cancer: Primary Care Management of patients at risk of breast, ovarian or colorectal cancer (Watch this Space!!) Based on NICE and BSG guidelines OPERA – tool for patients via MacMillan website The future! User-friendly family history tool linked to risk calculator in Primary Care Systems Use of google health - on-line family history questionnaires which can be accessed by health care professionals Useful information to include in referral: Usual personal details Whatever family history available Pedigree number / Name(s) of affected family members if seen by any genetics team (Pregnant or non-pregnant) Genetic Services Yorkshire Regional Genetic Service - very accessible! (based at CAH: switchboard 0113 243 2799) Covers Yorkshire and Humberside Medical Staff: Consultants, Registrars, GPwSI Counselling and diagnostic role Sub-specialise Cancer Genetics Team: Geneticist, Oncologist, GPwSI Genetic Counsellors - counselling role Family History Administrators - collate info DNA / Cytology labs - testing and research What happens when a referral is made? Referral received (can be via secondary care) Questionnaire Information collated by Family History Administrators Consultants/ GPwSI review Questionnaire Triage to Genetic Counsellor / Consultant / GPwSI Genetic Counselling (Peter Rose) Information gathering: Information provision: Discuss family history Identify patient concerns / wishes Explain risks and genetic contribution Discuss screening if appropriate Preventative measures Discuss tests if appropriate Decision making: Guide patient through difficult choices Institute management which patient chooses Genetic Counselling is NON-DIRECTIVE and NON-JUDGEMENTAL Doesn’t always result in a test! ‘Genetic Counselling is the process by which patients or relatives at risk of a disorder which may be hereditary are advised of the consequences of the disorder, the probability of developing or transmitting it and the ways in which this may be prevented or avoided’ Familial / hereditary cancer Population risk: should be reassured and managed in Primary Care Moderate risk (i.e. above population risk): Can be managed in secondary care Additional screening (annual mammogram from 40) NBSP ‘Typical’ patient no. 1 – Mrs. B High risk for breast and ovarian cancer Offered screening: Offered risk-reducing measures: Additional mammograms from age 40 MRI if mutation carrier or at 50% risk Ovarian screening likely to be proven ineffective Prophylactic Bilateral Mastectomy Prophylactic Bilateral Oophorectomy Offered testing: Given information and testing of affected relative discussed Prophylactic Tamoxifen… To test or not to test… Can inform risk Can reduce uncertainty May reassure May indicate if at risk of linked cancers - improve vigilance May alter access to screening Can be used as a decision aid re prophylactic surgery Can allow access to testing for unaffected family members – PREDICTIVE TESTING Cannot always give definitive result Cannot predict whether someone will or won’t get cancer Cannot predict when or how cancer may present Psychosocial consequences May not be needed to access screening / surgery May have implications for insurance applications A ‘typical’ morning surgery… Mrs T. attends, and after telling you about her athlete’s foot she bursts into tears and tells you her mother has just been diagnosed with cancer – ‘everyone in the family has it and I’m bound to get it’ What else do you ask? She tells you: Mother had breast cancer aged 64 Sister had cervical cancer in her 30’s Paternal grandfather had prostate cancer and died in his 80’s Her uncle developed lung cancer in his 60’s – he had been a heavy smoker all his life Role of Primary Care (NICE 2006) Women at or near population risk should be cared for in Primary Care They should receive standard information (see box in PACE Guidelines) ‘Be Breast Aware’ (NHS Breast Screening Programme and Cancer Research UK) ‘Are you worried about Breast Cancer?’ (www.macmillan.org.uk/cancerinformation) Communication… How would you try to reassure her that she wasn’t at any greater risk than the rest of the population? Contraceptive / HRT issues COCP: breast ca risk similar in those with or without family history (NICE, UKMEC 1) Reduced ovarian ca risk but not an indication BRCA1 mutation: 20% risk in ever use of COCP Preferable to use non-hormonal methods, or PO with lowest systemic dose (i.e POP/IUS) HRT: breast ca risk similar in those with or without family history (Million Women Study) Aled Maud CRC@58 Sian Huw Browyn CRC@57 CRC@54 50 Aled Olwen Dai Wynn Tom 37 34 34 33 29 John Died young ?renal Ca Jenny 40 Jane 20 Margaret Renal Ca@42 Roy Renal Ca@50 John 38 Richard Mark 35 33 Julian Becky Lily 18 16 13 Pat 58 Judith 30 28 Other resources for patients www.macmillan.org.uk/cancerinformation 0808 808 0000 www.cancerhelp.org.uk www.cctrust.org.uk 020 7704 1137 http://www.macmillan.org.uk/Get_Support/ Cancer_types/Genetic_risk_factors.aspx Can access OPERA via macmillan website The story so far… Our job is to identify the 1 in 20 patients with cancer (and their relatives) with a genetic basis (those in the red blob) PACE guidelines can help Genetic testing is only one aspect of management – also comprises information giving, extra screening, risk-reducing surgery. A typical morning surgery… Miss T: A 34-year-old lady with a history of depression comes to see you. Her sister died very suddenly 2 weeks ago at the age of 42. She also happens to be your patient, and when you look in her notes, the cause of death from PM is Myocardial Infarction Familial Hypercholesterolaemia 1 in 500 people have Familial Hypercholesterolaemia 50% CVD risk by the age of 50 in men 30% CVD risk by the age of 60 in women 110,000 cases in the U.K. Only around 10,000 identified so far When to think about it: Simon Broome Diagnostic Criteria TC >7.5, LDL >4.9 AND Definite FH: Tendon xanthomas in 1st or 2nd degree relative Possible FH: Family history of IHD <60 y.o.a. in 1st degree relative, and <50 y.o.a. in 2nd degree relative Family history of TC >7.5 in 1st or 2nd degree relative Cholesterol deposition in patients heterozygous for familial hypercholesterolemia. (a, b) Tendon xanthomata, and (c) corneal arcus. Fig. Disease box 11 ©Scion Publishing Ltd Photos courtesy of Dr Paul Durrington. How to manage it: Manage other risk factors Aggressively control cholesterol to lower LDL <50% level at initial measurement (treatment algorithm in NICE guidelines) If not controlled with 2 agents, refer: Donald Whitelaw (Diabetes Consultant, BRI) Andy Pettit (Diabetes Consultant, AGH) Julian Barth / Mike Mansfield (Lipid clinic, LGI) Assess for symptoms of IHD What about the Genetics? Autosomal Dominant Mutation in LDL receptor gene Cascade screening. Genotyping not widely available Role of primary care? The story so far… If someone has a family history of premature heart disease or presents with a cholesterol over 7.5: Think Familial Hypercholesterolaemia Other inherited cardiac conditions Specialist clinic at LGI FH of sudden cardiac death FH of arrythmia, cardiomyopathy or connective tissue disease Can refer directly Any queries: Kath Ashcroft 0113 3925784 or mobile 07789003997 A typical morning surgery… A ‘typical’ morning surgery… A 36-year-old man comes in ‘tired all the time’. He has several non-specific symptoms including palpitations and general aches, and you are aware he is having a stressful time at work. He is concerned, and asks you to do some blood tests. Hereditary haemochromatosis High index of suspicion in younger men who present TATT. Autosomal recessive disorders, carrier rate 1/8 – 1/10, prevalence 1/200 – 1/400. Signs, Symptoms and Complications: Non-specific – tatt, joint pain, weight loss, (impotence) Liver disease Diabetes Hypogonadotrophic hypognadism Arthritis Cardiac Disease (heart failure) Venesection improves life expectancy - normal if before development of diabetes and liver cirrhosis Hereditary haemochromatosis His ferritin came back as 458. What would you do next? Diagnosis: Ferritin: will be raised once iron overload occurring. Can also be raised in acute phase response If ferritin high, or high index of suspicion consider checking Fasting Transferrin (earliest marker of HH) If transferrin > 45%, refer to haematologist Generally, females >50%, males >55% Hereditary Haemochromatosis Genetics: 2 mutations responsible for >95% in U.K. Many people who inherit the mutations will not develop clinical disease. Genetics dept will offer gene testing / genotyping to 1st degree relatives only. A typical morning surgery… Mr. S, patient no 4. A 46-year-old gentleman comes in, and places a report in front of you. It lists his lifetime risk of many common conditions, but he is particularly concerned about his 45% lifetime risk of atrial fibrillation. Genomics Testing the ‘whole’ genome Population-based studies identify variants which have increased frequency in individuals who have a particular condition No assumption re mode of inheritance No prior knowledge of molecular mechanism needed Detect genetic mutation with small effect Research: map loci / possible mechanisms Susceptibility loci The position of these variants is then mapped to a particular locus – ‘susceptibility loci’ Increase likelihood of developing a particular condition But aren’t necessary or sufficient Personalised medicine? Limited applications – but its coming! Pharmacogenetics (azathioprine) Tumour profiling (Herceptin) Potentially used to increase risk stratification e.g. breast / prostate ca screening Phenotypic data still better predictors How would you manage Mr. S? Manage risk factors Manage concerns ELSI implications Genetic fatalism or change in behaviour? Other examples! A 24-year-old man who is diagnosed with Type 2 Diabetes. He has a normal BMI, is caucasian, and has no family history. A 59-year-old man who is caring for his wife who has just been diagnosed at 57 with early onset Alzheimer’s. Her mother also had dementia of some sort. Time for tea! Pedigree Symbols Male Marriage / Partnership (horizontal line) Female / Partnership that has ended Person whose sex is unknown P Offspring (vertical line) Pregnancy Miscarriage X weeks Affected Male & Female Carrier Male & Female Supporting Genetics Education for Health www.geneticseducation.nhs.uk Parents and Siblings Family History Jane (28) is 6 weeks pregnant Jane’s husband Christopher (29) is an only child His parents William (60) and Margaret (59) are alive and well Jane has one brother John (34), he had one son David (10) to his first wife Alice (33). Their marriage ended in divorce John’s second wife Christine (29) had a miscarriage at 9 weeks and a son Richard (4) who has CF Jane’s father George Whitehead died at the age of 66 Jane’s mother Joan (64) is alive and well Supporting Genetics Education for Health www.geneticseducation.nhs.uk Joan William Hobson 60 Margaret George Whitehead Died age 66 59 Christopher Hobson 29 Jane 28 Joan 64 Alice 33 John Whitehead 34 P 6 weeks Christine 29 9 weeks David 10 Assume Jane was tested and found to be a carrier. Richard 4 Cystic fibrosis What is the probability that the baby in Jane and Christopher Hobson’s current pregnancy will have cystic fibrosis? (Population risk of being CF carrier for people with North European Supporting Genetics Education for Health www.geneticseducation.nhs.uk ancestry = 1 in 25) This is the slide to remember! Our role is identify patients at risk or who may have a genetic condition and would benefit from input from Genetic Services We do this by taking and using a family history – core examples: A common multifactorial disease (e.g. IHD or cancer) occurring young, strong family history, atypical presentation Early pregnancy, or even pre-conceptually There is lots of information out there regarding individual conditions www.geneticseducation.nhs.uk Supporting Genetics Education for Health www.geneticseducation.nhs.uk Resources: Me! [email protected] YGS via LGI switchboard: 0113 243 2799 www.gpnotebook.com www.geneticseducation.nhs.uk ‘Recognising the common patterns of inheritance in families’ www.library.nhs.uk/genepool www.chime.ucl.ac.uk (apogi sheets Resources for patients www.geneticalliance.org.uk SWANUK [email protected] www.cafamily.org.uk Support for families in which there is a rare genetic disorder Thank you! Any questions? Family History Jane has one brother John (34) Jane and John’s father George Whitehead died at the age of 66 Jane and John’s mother Joan (64) is alive and well Supporting Genetics Education for Health www.geneticseducation.nhs. uk William Hobson 60 Margaret 59 Christopher Hobson 29 Jane 28 P 6 weeks Supporting Genetics Education for Health www.geneticseducation.nhs. uk George Whitehead Died age 66 Joan 64 John Whitehead 34 Family History Jane’s brother John has one son David (10) to his first wife Alice (33). Their marriage ended in divorce Supporting Genetics Education for Health www.geneticseducation.nhs. uk William Hobson 60 Margaret 59 Christopher Hobson 29 Joan 64 George Whitehead Died age 66 Jane 28 Alice 33 John Whitehead 34 P 6 weeks Supporting Genetics Education for Health www.geneticseducation.nhs. uk David 10 Family History John’s second wife is Christine (29) Christine had a miscarriage at 9 weeks They then had a son Richard (4) who has cystic fibrosis Supporting Genetics Education for Health www.geneticseducation.nhs. uk William Hobson 60 Margaret 59 Christopher Hobson 29 Joan 64 George Whitehead Died age 66 Jane 28 Alice 33 John Whitehead 34 P 6 weeks Supporting Genetics Education for Health www.geneticseducation.nhs. uk Christine 29 9 weeks David 10 Richard 4 Cystic Fibrosis William Hobson 60 Margaret 59 Christopher Hobson 29 George Whitehead Died age 66 Jane 28 Joan 64 Alice 33 John Whitehead 34 P 6 weeks Christine 29 9 weeks David 10 Richard 4 Cystic fibrosis From the family pattern, who must be carriers for cystic Supporting Genetics fibrosis? Education for Health www.geneticseducation.nhs. uk Supporting Genetics Education for Health www.geneticseducation.nhs. uk William Hobson 60 Margaret 59 George Whitehead Died age 66 Christopher Hobson 29 Jane 28 Joan 64 Alice 33 John Whitehead 34 Christine 29 or P 9 weeks 6 weeks David 10 Richard 4 Cystic fibrosis Is the probability of Jane Hobson being a carrier for cystic fibrosis sufficiently high to offer testing? Supporting Genetics Education for Health www.geneticseducation.nhs. uk Supporting Genetics Education for Health www.geneticseducation.nhs.uk Joan William Hobson 60 Margaret George Whitehead Died age 66 59 Christopher Hobson 29 Jane 28 Joan 64 Alice 33 John Whitehead 34 P 6 weeks Christine 29 9 weeks David 10 Assume Jane was tested and found to be a carrier. Richard 4 Cystic fibrosis What is the probability that the baby in Jane and Christopher Hobson’s current pregnancy will have cystic fibrosis? Supporting Genetics Education(Population for Health risk of being CF carrier for people with North European www.geneticseducation.nhs. ancestry = 1 in 25) uk Jane’s risk of being a carrier 1 X X Christopher’s risk of being a carrier 1 25 X X Chance of passing on two copies of gene change for CF 1 4 Supporting Genetics Education for Health www.geneticseducation.nhs.uk = = Risk of baby being affected by CF 1 100 Joan William Hobson 60 Margaret George Whitehead Died age 66 59 Christopher Hobson 29 Jane 28 Joan 64 Alice 33 John Whitehead 34 P 6 weeks 9 weeks David 10 When should Genetic advice be sought? Which other family members should be offered carrier status testing? Supporting Genetics Education for Health www.geneticseducation.nhs. uk Christine 29 Richard 4 Cystic fibrosis Consanguineous Marriage What is Consanguinity WHO defines consanguineous marriage as one between individuals who are second cousins or more closely related. Consanguinity comes from the Latin words: con meaning shared and sanguis meaning blood. The global distribution of consanguineous marriage History of Consanguineous Marriage in Europe Consanguineous marriage was prevalent until the 20th century, and was associated with royalty and landowning families Brain Storm! What are the perceived benefits and disadvantages of cousin marriages? Perceived benefits of cousin marriages Keeping property and money within a family Staying within a well understood family unit Improving the position of women by reducing the chances of mistreatment from a husband bound by family ties. Perceived Disadvantages of cousin marriages Risk of genetic conditions in offspring Anxiety about social stigma Difficulty living an autonomous married life separate from the wider family Why is there an offspring risk associated with consanguinity? When gametes formed, a few alterations in the DNA code will occur – usually result in healthy carrier state. Only when two people who carry the same alteration / mutation reproduce is there an increased chance of autosomal recessive disease This occurs in conditions with a higher carrier rate in the general population, people of similar ethnicity, and people who are related A related couple are more likely to have an alteration in the same gene because they have both inherited some of their genes from their shared relatives. In the case of first cousins, both of them could have inherited the same changed gene from one of the grandparents that they share. Consanguineous couples are therefore at increased risk Autosomal Recessive Inheritance Risk Of Having a Child with Severe Congenital or Genetic Disorder Diagnosed by 1 yr of Age Unrelated parents 2-3% First cousins 4-5% Second cousin 3-4% Double first cousins 6-7% BUT also increased with maternal age, smoking, drinking, drugs, poor nutrition, poor obstetric/healthcare Risk of having a child with disability Risk for first cousins is still low (i.e. 4% instead of 2%, 96% have healthy children) but this is doubled, not a 2% increase Risk for the community is an extra 2% incidence (i.e. with 2,000 consanguineous births each year, an extra 40 children with autosomal recessive conditions) Every Baby Matters Initiative in Bradford Bradford district Infant Mortality report: Review of data on births and deaths between 1995-03 and found that babies in Bradford: 1.7 times more likely to die in their first year than babies born in England and Wales as a whole. ( 2001-3 figures) more likely to die from congenital anomalies, infections and other specific conditions born to Pakistani-origin mothers were twice as likely to die in their first year of life compared to caucasian mothers as a whole – this increased burden of infant mortality is seen across England and Wales as well as in Bradford. 64 infant deaths across the District a year between 1996 and 2003 When compared with England as a whole, predicted total number of deaths 41 Approximate excess of 23 deaths a year in Bradford. Aim of Every Baby Matters Initiative in Bradford 10 Recommendations (Genetics no. 7) 58 % of Bradford’s births (approximately 5,000 a year) are from the two most deprived quintiles and our challenge is to decrease the numbers of babies that die within these births by 10%. ‘To make sure that all parents with one child with an inherited disorder will understand the risk of a future baby carrying a similar disorder, and be in a position to make an informed choice about having another baby.’ Info in Leaflet : Be genetics aware! Families from all communities can be affected by genetic disorders We know more about genes today than we used to, so it's right that we have access to information and services so we can make informed choices There are many health problems associated with genes but, in infant health, there is a particular concern with problems caused by recessive genes Common examples of these types of conditions include cystic fibrosis, sickle cell disease, thalassaemia and some neurological and metabolic diseases For conditions caused by recessive genes, the risk is higher in families with a marriage to a close relative, e.g. a cousin, as it is more likely they both carry the same gene It's important to note that most children born to cousins are healthy and unaffected, but babies born to parents who have the same recessive gene are at a higher risk of being born with an inherited health problem and some rare recessive conditions can prove fatal Talk to your GP if you think that a child in your own or your wider family may have been affected by an inherited disorder. Your GP can help you to assess the risks and if necessary book you an appointment with a genetic counsellor A genetic counsellor will be able to give a more rounded picture about your risk of genetic problems and what your choices are around this Counselling in primary care Explore what they know and want to know ‘Screen’ family history • Any history of known recessive disorder? Remember to ask about family history of: 1. 2. 3. 4. 5. Miscarriages and still births Birth defects (such as cleft lip, heart defects, spina bifida, limb abnormalities) Blindness/vision loss/deafness/hearing loss at a young age Developmental delay/ learning disorders Regular attendance at CDC / OPD or visits from outreach team e.g. Metablic Counselling in Primary Care If no significant family history: Can counsel re general risk Offer screening according to ethnicity No other specific testing possible If possible / significant family history: Are they pregnant? If pregnant and would affect decisions then refer urgently i.e. phone What do they want to know? Can offer routine referral otherwise. Resources for patients www.cafamily.org.uk Support for families in which there is a rare genetic disorder SWANUK [email protected]