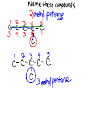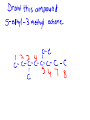* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download Naming Straight Chain Alkanes (C
Survey
Document related concepts
Transcript
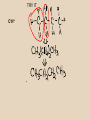
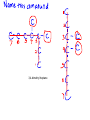
Organic Chemistry Chemistry of Carbon Compounds Why so many Carbon compounds?! Group #_14__________________ metal, nonmetal, metalloid bond type__covalent____ Valance electrons__4__ Lewis Dot formula Bonding Capacity__4____ C C C Single Bond C C Double Bond C C Triple Bond Bonding Capacity of H, O, N H O N 1 2 3 Hmmm, I seem to remember something about that....... Why covalent bonds? Electronegativity: ·The ability to pull electrons away from another element ·elements in the NE part of the periodic table have stronger pull METALS NONMETALS Properties of Covalent Compounds ·Covalent compounds generally have much lower melting and boiling points than ionic compounds. ·Covalent compounds don't conduct electricity in water. ·Covalent compounds aren't usually very soluble in water. ·Covalent compounds tend to be more flammable than ionic compounds. Chemical Formulas (Molecular formulas) CH4 methane one pair of electrons C2H6 H ethane H H C C H H Lewis Dot H C H H H H Structural Formulas H H C H each pair of electrons = one single bond H H H H C C H H H Condensed Structural Formula Propane Chemical (molecular) Formula C 3H 8 H H Structural Formula H C C C H Condensed Structural Formula H H H H CH3CH2CH3 same as structural just take away the lines! TRY IT C4H10 ANOTHER WAY C6H14 count how many CH2's there are HOMOLOGOUS SERIES ·a series of organic compounds with a similar general formula ·possessing similar chemical properties due to the presence of the same functional group, and ·shows a gradation in physical properties as a result of increase in molecular size and mass ALKANES ·straight-chain or branched-chain hydrocarbons in which all the bonds between carbon atoms are single covalent bonds. ·"saturated" ·general formula is ·end with the suffix " ane " CnH2n+2 where "n" is the # of carbons Naming Straight Chain Alkanes (CnH2n+2) Prefix is determined by the number of carbon atoms in the chain: Suffix is "ane" meth th prop but pent hex hept oct non dec e C1 C2 C3 C4 C5 C6 C7 C8 C9 C10 CH4 C2H6 Naming Branched Alkanes Functional Group Functional groups are specific atoms, ions, or groups of atoms having consistent properties. A functional group makes up part of a larger molecule. In branched alkanes the functional group in an "ALKYL" group CH3 methyl ethyl yl ending C2H5 one less Hydrogen! 3,4-dimethyl heptane Naming Branched Chain Alkanes http://www.ausetute.com.a u/nambanes.html · Name and number the longest carbon chain as for a straight chain alkane. · Identify each of the branches (side-chains). · Use the lowest number combinations for the branches (side-chains). · Name each branch or side-chain (alkyl group) : methyl CH3 ethyl C2H5 propyl C3H7 · For more than 1 of the same alkyl group use: di = 2 tri = 3 tetra = 4 · Use commas between numbers, eg, 1,2 or 2,3 · Use hyphens between numbers and words, eg, 2-methyl or 2,3-dimethyl · If there is more than 1 type of branch or side-chain, arrange their names alphabetically, ie, ethyl groups are named before methyl groups which are named before propyl groups