* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download presentation - Critical Path to TB Drug Regimens
Pharmaceutical marketing wikipedia , lookup
National Institute for Health and Care Excellence wikipedia , lookup
Specialty drugs in the United States wikipedia , lookup
Psychedelic therapy wikipedia , lookup
Drug design wikipedia , lookup
Polysubstance dependence wikipedia , lookup
Pharmacokinetics wikipedia , lookup
Orphan drug wikipedia , lookup
Drug discovery wikipedia , lookup
Neuropharmacology wikipedia , lookup
Pharmacognosy wikipedia , lookup
Neuropsychopharmacology wikipedia , lookup
Psychopharmacology wikipedia , lookup
Pharmacogenomics wikipedia , lookup
Drug interaction wikipedia , lookup
Cross-Sector Perspectives:
Global Health
CPTR Workshop
October 2-4, 2012
Christian Lienhardt
Stop TB Department
Overview of the presentation
• Background – Global TB burden
• Challenges in introducing new
TB drugs/regimens
• Developing Guidance to
countries
• A pluri-partners model
Overview of the presentation
• Background – Global TB burden
• Challenges in introducing new
TB drugs/regimens
• Developing Guidance to
countries
• A pluri-partners model
The Global Burden of TB -2010
Estimated TB incidence rates, by country, 2010
TB cases
per 100 000
0–24
Estimated
number of cases
25–49
50–99
100–299
>=300
No estimate
All forms of TB
HIV-associated TB
Multidrug-resistant
TB (MDR-TB)
8.8 million
(range: 8.5–9.2 million)
1.1 million (13%)
(range: 1.0–1.2 million)
440,000
(range: 390,000–510,000)
Estimated
number of deaths
1.4 million
(range: 1.2–1.6 million)
350,000
(range: 320,000–390,000)
about 150,000
Estimated TB incidence rates, by country, 2010
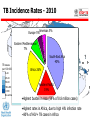
TB Incidence Rates - 2010
Americas 5%
Europe 5%
Eastern Mediterranean
7%
South-East Asia
40%
TB cases
per 100 000
Africa 26%
0–24
25–49
50–99
100–299
>=300
No estimate
Western Pacific
19%
•Highest burden in Asia (59% of 8.8 million cases)
•Highest rates in Africa, due to high HIV infection rate
~80% of HIV+ TB cases in Africa
Impact of HIV on TB in Africa
•80% of all TB/HIV cases world-wide are in Africa
•50% of all TB/HIV cases world-wide in 9 African countries
•23% of the estimated 2 million HIV deaths due to TB
Notified cases per 100,000 pop. 1980-2008
AFR
South Africa
700
SEA
India
Nigeria
600
Zimbabwe
Uganda
Kenya
Mozambique
500
Ethiopia
WPR
Zambia
400
AMR
United Republic of Tanzania
Malawi
300
Côte d'Ivoire
Botswana
Côte d'Ivoire
DR Congo
Gabon
Guinea
Kenya
Malawi
Mozambique
South Africa
UR Tanzania
Zimbabwe
EUR
Myanmar
200
China
Democratic Republic of the Congo
Brazil
Thailand
100
Cameroon
EMR
1%
5%
10%
20%
Percentage of global estimated HIV-positive TB cases
50%
90%
0
1980
1984
1988
1992
1996
2000
2004
2008
% MDR-TB among new TB cases,
1994-2009
0-<3
3-<6
6-<12
12-<18
>=18
No data available
Subnational data only
Australia, Democratic Republic of the Congo, Fiji, Guam, New Caledonia, Solomon
Islands and Qatar reported data on combined new and previously treated cases.
The boundaries and names shown and the designations used on this map do not imply the expression of any opinion whatsoever on the part of the World Health
Organization concerning the legal status of any country, territory, city or area or of its authorities, or concerning the delimitation of its frontiers or boundaries. Dotted lines on
maps represent approximate border lines for which there may not yet be full agreement.
WHO 2010. All rights reserved
Notified cases of MDR-TB
Estimated absolute numbers of reported
cases with MDR-TB*
Notified cases of MDR-TB
Cases of
MDR-TB
<100
0–99
100–999
100–999
1000–9999
1000–9999
≥10 000
>10,000
*among reported pulmonary TB patients
NA
13 top settings with highest % of MDR-TB
among new cases, 2001-2010
Preliminary results
Minsk, Belarus (2010)
35.3
28.3
Murmansk Oblast, Russian Federation (2008)
27.3
Pskov Oblast, Russian Federation (2008)
23.8
Arkhangelsk Oblast, Russian Federation (2008)
22.3
Baku city, Azerbaijan (2007)
20.0
Ivanovo Oblast, Russian Federation (2008)
Republic of Moldova (2006)
19.4
Kaliningrad Oblast, Russian Federation (2008)
19.3
Belgorod Oblast, Russian Federation (2008)
19.2
Dushanbe city and Rudaki district, Tajikistan (2009)
16.5
Mary El Republic, Russian Federation (2008)
16.1
Donetsk Oblast, Ukraine (2006)
16.0
Estonia (2008)
15.4
Tashkent, Uzbekistan (2005)
14.8
0
5
10
15
20
25
30
Countries that had reported at least one
XDR-TB case by late 2011
Argentina
Armenia
Australia
Austria
Azerbaijan
Bangladesh
Belgium
Botswana
Brazil
Burkina Faso
Bhutan
Cambodia
Canada
Chile
China
Colombia
Czech Republic
Ecuador
Egypt
Estonia
France
Georgia
Germany
Greece
India
Indonesia
Iran (Islamic Rep. of)
Ireland
Israel
Italy
Japan
Kazakhstan
Kenya
Kyrgyzstan
Latvia
Lesotho
Lithuania
Mexico
Mozambique
Myanmar
Namibia
Nepal
Netherlands
Norway
Pakistan
Peru
Philippines
Poland
Portugal
Qatar
Republic of Korea
Republic of Moldova
Romania
Russian Federation
Slovenia
South Africa
Spain
Swaziland
Sweden
Tajikistan
Thailand
Togo
Tunisia
Ukraine
United Arab Emirates
United Kingdom
United States of America
Uzbekistan
Viet Nam
The boundaries and names shown and the designations used on this map do not imply the expression of any opinion whatsoever on the part of the World Health
Organization concerning the legal status of any country, territory, city or area or of its authorities, or concerning the delimitation of its frontiers or boundaries.
Dotted lines on maps represent approximate border lines for which there may not yet be full agreement. WHO 2011. All rights reserved
The Global TB Control Targets
2015: Goal 6: Combat HIV/AIDS, malaria and other diseases
Target 6c: to have halted by 2015 and begun to reverse the incidence…
*Indicator 6.9: incidence, prevalence and mortality associated with TB
*Indicator 6.10: proportion of TB cases detected and cured under DOTS
2015: 50% reduction in TB prevalence and deaths by 2015
2050: elimination (<1 case per million population)
Rate per 100,000 population
Incidence, prevalence and mortality
rates: global estimates
Incidence
Prevalence
Mortality
150
100
250
25
200
20
150
15
100
10
Peak in 2002
50
Target
Target
50
5
Falling 1.4% per year
0
40% decline since 1990
0
1990
2010
0
1990
2015
1990
2015
Global notifications (black)
in context of estimated incidence (green)
8.8
TB cases (millions)
7.6
Detection gap: 1/3
5.8
3.7
Estimated Global
Case Detection
65%
(63–68%)
Treatment success 87% globally
…but Europe lagging behind
87
88
86
WHO Regions, new sm+
95
W. Pacific 93
90
SE Asia 89
86
85
86
84
83
84
82
EMR 88
85
Africa 81
80
80
20
09
20
08
20
07
65
20
06
76
20
05
Europe 67
20
04
70
20
03
78
20
09
Americas 76
20
06
20
07
20
08
75
20
04
20
05
80
20
03
Treatment success rate (%)
Global, new sm+
Notifications of MDR-TB increasing
BUT only ~ 1 in 5 (19%) of estimated cases of MDR-TB among
reported TB patients diagnosed and treated in 2011
MDR-TB cases treated and
estimated numbers not treated
for MDR-TB, among notified TB
patients, 2010
Notified cases of MDR-TB
60
290,000
300
50
53,000
250
Not on treatment
Treated
40
200
30
150
20
100
2008
2009
2010
or
ld
2007
W
2006
Am R
er
ic
as
2005
EM
0
Af
r
0
A
si
W
.P a
ac
ifi
c
Eu
ro
pe
50
ic
a
19,000
10
SE
Number of patients (thousands)
Global Plan target ~270,000 in 2015
Time trends in TB and MDR-TB:
reverting, controlling, and alarming…
____ TB ____ MDR-TB
100
____ TB
-6.7% per year
____ MDR-TB
100
2.4% per year
10
10
-2.4% per year
-5.1% per year
1
1998
1
2000
2002
2004
2006
2008
1999
2001
2003
2007
2009
Tomsk Oblast, Russia
Estonia
____ TB ____ MDR-TB
1000
0.3% per year
100
10
Botswana
2005
19.4% per year
1
1996
1998
2000
2002
2004
2006
2008
Overview of the presentation
• Background – Global TB burden
• Challenges in introducing new
TB drugs/regimens
• Developing Guidance to
countries
• A pluri-partners model
Current TB Therapy and Unmet Needs
Patient Population
Current Therapy
Unmet Needs
Drug-Susceptible
DS-TB
4 drugs; 6 month therapy
(2RHZE + 4RH)
Shorter, simpler therapy
Drug-Resistant
M(X)DR-TB
At least 4 drugs (including
injectable); ≥18 months;
poorly tolerated
Fully oral, shorter and
safer therapy
TB/HIV
co-Infection
Drug-drug interactions (DDI)
with ARVs
No or low DDI, coadministration with ARVs
Latent TB
Infection
6-9 months H
Shorter, safer therapy
* Rifampin (R), Isoniazid (H), Pyrazinamide (Z), Ethambutol (E)
► For all indications and treatment, issues in delivery and access
► Need shorter and simpler therapies against both DS and DR-TB
Adapted from TB Alliance
Global TB Drug Pipeline 2012
Discovery1
Lead Optimization
Nitroimidazoles
Mycobacterial Gyrase
Inhibitors
Riminophenazines
Diarylquinoline
Translocase-1
Inhibitor
MGyrX1 inhibitor
InhA Inhibitor
GyrB inhibitor
LeuRS Inhibitor
Pyrazinamide
Analogs
Spectinamides
Preclinical Development
Preclinical
Development
CPZEN-45
SQ641
SQ609
DC-159a
Q201
Clinical Development
GLP
Tox.
BTZ043
Phase I
AZD5847
Phase II
Bedaquiline (TMC207)
PA-824
Linezolid
SQ-109
Rifapentine
Novel Regimens2
PNU-100480
Phase III
Gatifloxacin
Moxifloxacin
Rifapentine
Delamanid (OPC67683)
Chemical classes: fluoroquinolone, rifamycin, oxazolidinone, nitroimidazole, diarylquinoline, benzothiazinone
1 Ongoing
projects without a lead compound series can be viewed at
http://www.newtbdrugs.org/pipeline-discovery.php
2
Combination regimens: first clinical trial (NC001) of a novel TB drug regimen testing the three drug combination of PA-824,
moxifloxacin, and pyrazinamide completed August 2011.
www.newtbdrugs.org
Public health challenges of introduction of
new TB drugs in countries
•
Novel drugs and shortened treatment regimens with new and/or
re-purposed drugs are proceeding along the clinical development
pathway;
•
New drugs submitted for regulatory approval for treatment of
drug-resistant MDR-TB;
•
Implications for TB control programmes:
– determine optimal regimens for use of newly developed and/or
repurposed drugs for treatment of DS- and DR-TB under
programmatic conditions;
– evaluate requirements for patients’ eligibility;
– assess programmatic feasibility;
– evaluate cost-effectiveness of newly-developed treatments;
– ensure proper surveillance and pharmacovigilance;
– ensure responsible use (appropriate indication, doses, drug
combination(s), and treatment duration) – prevent off-label use
and amplification of resistance;
Overview of the presentation
• Background – Global TB burden
• Challenges in introducing new
TB drugs/regimens
• Developing guidance to
countries
• A pluri-partners model
STAG-TB recommendations for guidance
on new drugs for TB (Sept 2010)
STAG-TB recommends that:
-
WHO issues clear policies to guide countries on the introduction of
new regimens for treatment of DS and DR-TB […] upon availability of
evidence in support of use of such regimens;
-
WHO issues specific requirements on what evidence and information
would be needed to develop policy recommendations related to new
drugs/regimens for treatment of DS and DR-TB;
-
WHO promotes collaboration and action by partners [...], so that
appropriate drug regimens are utilized by programmes for the
treatment of DS- and DR-TB, inclusive of the new drugs, and avoid
irrational use of new tools;
-
WHO organizes expert consultation(s) to review the evidence for use
of new drugs and regimens to inform timely development of treatment
policy guidance to national health authorities.
Task Force for new TB drug policy development
Objectives:
• To advise and assist WHO Stop TB in the development and
monitoring of a strategic plan to prepare WHO policy guidance
for the rational introduction of new TB drugs/regimens in
countries;
• To advise and assist in the development of a policy development
framework for the introduction of new drugs/regimens for the
treatment of TB in countries;
• To assist and facilitate the implementation and evaluation of
activities listed in the strategic plan
Established April 2012; 12 members + 2 observers
The Strategy Plan - Principles
• Introduction of new anti-TB drugs in practice is a multistage process;
• Development of appropriate combination(s) of drugs needs efficient
coordination and sharing of data between key partners;
• Introduction of new TB drugs should be adaptable to countries settings
according to country's own health infrastructure and preparedness;
• Need for rapid approval of new TB drugs by regulatory authorities in
high-TB burden countries so as to favor due access;
• Equitable access to new drugs to all patients in needs worldwide is
essential and should be linked with measures to prevent misuse of the
drugs;
• WHO has a key role to play for the development of policy
recommendations for rational introduction and use of new
drugs/regimens in programmatic settings, ensuring proper, equitable
and cost-effective access to treatment.
The Strategy Plan – Contents (1)
I. Determination of the type of evidence and data that would be
required by WHO to recommend the use of new drug(s)/
regimen(s) for the treatment of DS and/or MDR-TB
• Review of current drug/regimen development landscape
• Review of requirements for licensure by stringent regulatory
authorities
• Determination of data according to indications (DS or DR-TB)
and populations (children, PLHIV)
• Priority categories (fundamental -> nice to have)
The Strategy Plan – Contents (2)
II. Production of information notes:
• aimed at facilitating the production of policy
recommendations for the treatment of TB (all forms),
according to progress made in the development of new
drugs/combinations of drugs
• Information notes:
– to countries
– to drug/regimen developers
– to regulators
– on compassionate use
The Strategy Plan – Contents (3)
III. Development of a "Policy Development Framework” for the
introduction of new TB drugs/regimens in countries.
• Describes the process for development of policies for treatment of TB
including the new drugs/regimens.
• Will be used to guide development of policy recommendations for
specific drugs/regimens as data become available
– based on progress of new drugs/drug combinations along clinical
development pathway (e.g. PK and drug-drug interaction studies, Phase II
trials, Phase III pivotal trials),
– approval by stringent regulatory authorities (FDA, EMA, …).
• Will be used by the expert committees that will be convened by WHO
to update/revise policy recommendations as needed.
The Strategy Plan – Contents (4)
IV. Plan for expert consultations on revision of treatment
guidelines (depending upon drug pipeline development)
•
•
•
•
•
•
Timing of meetings (through 2012-2014)
Drugs/regimens to be assessed
Preparation of key data
Experts selection & planning
Discuss with GRC - Rapid advice approach ?
Include time for STAG submission/endorsement and finalization of
policies
The Strategy Plan – Contents (5)
V. Market introduction
• map-out the detailed expertise needed (drug market
introduction, pricing, funding, public vs. private issues) and
identify appropriate stakeholders (incl. GF; UNITAID; GDF; CHAI,
etc)
• Evaluate market shortcomings and commodity access issues
• Identify potential obstacles related to introduction and work
with stakeholders (countries, drug developers, economists,
market specialists, NGOs, donors…) to optimize market
introduction.
The Strategy Plan – Contents (6)
VI. Introduction in countries
• Country preparedness
– Background epidemiological data ("know your epidemics")
– Health infrastructure
– NTP structure
• Country support to enable access to new drugs
– Strengthened capacity for diagnosis, drug resistance surveillance,
pharmacovigilance
– Standardized definitions of outcomes (harmonize data collection after drug
introduction)
– Drug supply and management
– Discuss check/control mechanisms (accreditation)
– Develop "Demonstration sites" for initial deployment of new drugs with
harmonised methods and surveillance
Overview of the presentation
•
Background – Global TB burden
•
Challenges in introducing new
TB drugs/regimens
•
Developing Guidance to
countries
•
A pluri-partners model
Key messages
•
Ensure equitable access to new drugs to all patients in needs
worldwide and avoid acquisition of new resistances
•
Identify suitable drug combination(s) for treatment of DS and
DRTB early
•
Need to work on country preparedness and clarify conditions
for controlled/accredited access to new drugs
•
Encourage collaboration between drug developers, regulators,
and programme managers
•
Find the suitable balance for easy access to new therapies and
guarantee patients protection with use of drugs that remain
efficient and safe worldwide
A pluri-partners model
• Dialogue needed with drug/regimen developers, sponsors,
regulators, National TB Programme Managers.
• Information Notes:
– Drafts circulated to partners/stakeholders for comments
and feed-back to ensure buy-in
– To be finalised and disseminated in Oct-Dec 2012
• Meetings with stakeholders:
– Task Force
– Satellite meeting at International Conference of Drug
Regulatory Authorities (ICDRA), Tallinn (22 Oct 2012)
– Meeting at International Conference on Lung Health,
Kuala Lumpur (Nov 2012)
A role for CPTR
• As unique forum grouping representatives from key drug
development initiatives, CPTR has a critical role to play in
the dialogue with key stakeholders.
• Contribute/promote interactions – e.g. through
- Global Regulatory Pathway Working Group
- Working Group on Access and Appropriate Use
• Contribute to works of the Task Force for New Drug Policy
Development – technical resource
• Later stages ?
Acknowledgements
•
Task Force members
•
WHO staff, particularly Samvel Azatyan, Dennis Falzon,
Christopher Fitzpatrick, Malgosia Grzemska, Ernesto Jaramillo,
Shanthi Pal, Andrea Pantoja, Charles Penn, Lembit Rägo, Mario
Raviglione, Diana Weil & Karin Weyer
•
BMGF; USAID; DfID
Thank you for
your attention !