* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download Lesson 7.8 Basic Properties of the Main Group Elements Suggested
Alkali metal wikipedia , lookup
Group 12 element wikipedia , lookup
Carbon group wikipedia , lookup
Boron group wikipedia , lookup
Alkaline earth metal wikipedia , lookup
Period 6 element wikipedia , lookup
Group 3 element wikipedia , lookup
Period 5 element wikipedia , lookup
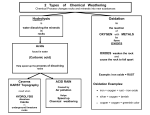
Lesson 7.8 Basic Properties of the Main Group Elements Suggested Reading Zumdahl Chapter 7 Section 7.13 Essential Question What are the basic properties of the main group elements? Learning Objectives Describe the basic characteristics of the main-group elements. Discuss the similarities and differences in the chemical properties of elements in the same group. Introduction In this lesson we will look at the periodicity of chemical properties of the main-group elements (s & p block elements). However, before we can do this we must review the metallic character of the elements and the properties of oxides. Metallic Character When looking at the basic characteristics of the main-group elements, it is important to recall how metallic/nonmetallic character of the elements changes going through the periodic table. Metallic elements lie to the left of the "staircase" (amphoteric line), nonmetals lie to the right and the metalloids (with intermediate characteristics) lie along the staircase. Thus the elements tend to increase in metallic character in going from right to left in any period. The elements also tend to increase in metallic character going down any column of elements. Some groups show this tendency more clearly than others. For example, Group IVA starts with carbon (nonmetal), and goes to silicon and germanium (metalloids), and concludes with tin and lead (metal). The trends in metallic/nonmetallic character should roughly follow the trends in ionization energies. The situation with electron affinities is less straightforward, but you can say that the reactive nonmetals have large negative electron affinities. Recall from the previous lesson that large negative electron affinities indicate a very stable negative ion is formed. These substances are the elements in Groups VIA and VIIA. Although the elements in a given group are expected to be similar, the degree of similarity varies. The alkali metal elements show marked similarities to one another, as do the halogens. On the other hand, the Group IVA elements range from a nonmetal at the top of the column to a metal at the bottom. The periodic table helps you to understand these systematic changes. Most main-group elements from compounds with oxygen. Because the basic/acidic behavior of the oxides is a good indicator of the metallic/nonmetallic character of the elements, we need to look at the general behavior of the oxides. Basic and Acidic Oxides Oxides can be divided into two main types, basic oxides and acidic oxides, depending on their reaction with acids and bases. A basic oxide (basic anhydride) is an oxide that reacts with acids. Most metal oxides are basic oxides. The soluble ionic oxides give basic solutions with water to give the OH- ion and therefore a basic solution (According to the solubility rules, all oxides are insoluble EXCEPT those of calcium, barium and Alkali metal (Group I) cations.). CaO(s) + H2O(l) → Ca2+(aq) + 2OH-(aq) Many metal oxides are not soluble in water but do react with acids; they are therefore basic oxides. Nickel(II) oxide, NiO, is an example. NiO(s) + 2H+(aq) → Ni2+(aq) + 2H2O(aq) These equations provide an excellent example of our different views of bases. Note how the definition of an Arrhenius base applies to the the first reaction while the Bronsted-Lowry definition applies to the second equation. An acidic oxide (or acidic anhydride) is an oxide that reacts with bases. Most nonmetal oxides are acidic oxides. The soluble acidic oxides dissolve in water to produce acidic solutions. Carbon dioxide, for example, dissolves in water to give unstable carbonic acid, (H2CO3). CO2(g) + H2O(l) ⇌ H2CO3(aq) Silicon dioxide (silica, glass) is an inert solid in water, but when fused (melted) with a base or basic oxide, it gives a salt, and so it acts like an acidic oxide. For example, SiO2(s) + CaO(s) + ∆ → CaSiO3(l) (calcium silicate) Now that we have reviewed metallic character and oxides, we can discuss the main-group elements. We will look at some of the major characteristic of these elements, paying particular attention to the oxides. In the case of the nonmetals, we will also examine the compounds with hydrogen. You may want to make flash cards to help you remember the major characteristics of the elements we are about to discuss. You will be tested on these characteristics on in class, AP, and IB exams. Hydrogen (1s1) Although he electron configuration of hydrogen is like that of the alkali metals, its physical and chemical properties are quite different. The element is a colorless, odorless gas composed of H2 molecules. It is not reactive with water but does burn in oxygen. 2H2(g) + O2(g) → 2H2O(g) At very high pressures, however, hydrogen is believed to have metallic properties. Group IA Elements, the Alkali Metals (ns1) YouTube Video The alkali metals are soft and reactive. All react with water. For example, 2Li(s) + 2H2O(l) → 2LiOH(aq) + H2(g) The reactivity increases going down the group of elements. When a small piece of lithium is added to water, it moves about the surface, evolving hydrogen. Sodium reacts similarly, though hydrogen is evolved more rapidly. The release of heat is also noticeable; it may ignite the hydrogen! In the case of potassium, the reaction is quite vigorous, and the heat of reaction ignites the hydrogen to produce a flame. All alkali metals can form basic oxides with the general formula R2O. Watch this You Tube video https://www.youtube.com/watch?v=l9z5-mJ8NZk Group IIA Elements, the Alkaline Earth Metals (ns2) The alkaline earth metals are also reactive, but much less so than the alkali metals. Reactivity increases going down a group. Beryllium shows no reaction with water while magnesium reacts very slowly. Calcium, strontium, and barium react readily with water. Ca(s) + 2H2O(l) → 2Ca(OH)2(aq) + H2(g) All alkaline earth elements form basic oxides with the general formula RO. Group IIIA Elements (ns2np1) The Group IA and Group IIA elements are all relatively reactive metals, and there is only a slight variation in metallic character. Within the group IIIA elements, we see a significant increase in metallic character going down the column of elements. The first Group IIIA element, boron, is a metalloid having several allotropes, or different forms of the element. In one form, boron is a black, crystalline substance with a metallic luster. Boric acid, (B(OH)3 or H3BO3), is a common boron compound used as a mild antiseptic. Heating boric acid yields boric oxide, B2O3, an acidic oxide used to make heat-resistant glass (Pyrex). Other elements in this group are metals and form oxides with the general formula R2O3. Aluminum is a common structural material. Aluminum oxide, Al2O3, is an amphoteric oxide. An amphoteric substance is a substance that has both acidic and basic properties. Aluminum oxide dissolves in acids to produce aluminum ion, as expected for a basic oxide. Al2O3(s) + 6H+(aq) → 2Al3+(aq) + 3H2O(l) The oxide also dissolves in strong bases, as expected for an acidic oxide. Al2O3(s) + 3H2O(l) + 2OH-(aq) → 2Al(OH)4-(aq) The metal underneath aluminum is gallium, which metals when held in the palm of your hand. Gallium forms the amphoteric oxide, Ga2O3. Indium and thallium are metals with basic oxides, In2O3 and Tl2O3. Note the trend in the Group IIIA elements from acidic to amphoteric to basic oxides, which follows the trend of greater metallic character as we go down a column. Group IVA Elements (ns2np2) YouTube Video We have already mentioned the distinct trend from nonmetal to metal going down the Group IVA elements. Carbon Exists in two well-known allotropic forms: graphite, a soft, black substance used in pencil leads and diamond, a very hard, clear, crystalline substance. Another allotrope a carbon is a molecule buckministerfullerene,C60, which has a cage-like fused ring structures that form buckyballs and nanotubes. Both tin and lead have been known and used by humans since ancient times. Tin is alloyed, or mixed with copper to make bronze, which contains about 90% copper and 10% tin. Some believe that lead acetate, which was used as a sweetener by ancient Romans, brought down the entire empire! Here is a link to in article about this (optional, but interesting, reading). All of the elements in this group form oxides with the general formula, RO2. Carbon dioxide is a gas and an acidic oxide. Carbon also forms a stable monoxide, CO. Silicon dioxide, SiO2, an acidic oxide, exists as quartz and with sand (particles of quartz). Amphoteric tin dioxide, SnO2, is found in the mineral cassiterite, the principle ore of tin. Lead forms, PbO2, and the monoxide PbO, which are both amphoteric oxides. Lead monoxide is more stable. Watch this You Tube video https://www.youtube.com/watch?v=Kl083LAYnoU Group VA Elements (ns2np3) Group VA elements also show the transition from nonmetal (nitrogen, N, and phosphorus, P), to metalloid (arsenic, As, and antimony, Sb) to metal (bismuth, Bi). Nitrogen occurs as a colorless, odorless gas with N2 molecules. It is a relatively unreactive element. White phosphorus is a white, waxy solid with P4 molecules. It is quite reactive and is normally stored under water. In air it bursts into flame reacting with O2 to give the acidic oxides P4O6 and P4O10. Other allotropes, red and black phosphorus are much less reactive. Grey arsenic, the common form of the element, is a brittle solid with metallic luster. Yellow arsenic is an unstable, crystalline solid composed of As4 molecules. Antimony is a brittle solid with a silvery, metallic luster. Bismuth is a hard lustrous metal with a pinkish tinge. Group VA elements form oxides with empirical formulas R2O3 and R2O5. In some cases the molecular formulas are twice these formulas, as in the case of phosphorus. Nitrogen has the acidic oxides N2O3 and N2O5, although it has other better known oxides. Nitric oxide, NO, is a gas produced as a byproduct of combustion when nitrogen reacts with oxygen. Many people take supplements of this oxide, because is also an important cellular signaling molecule is mammals. Nitric oxide combines readily with more oxygen to give nitrogen dioxide, NO2, a brown gas. N2(g) + O2(g) → 2NO(g) 2NO(g) + O2(g) → 2NO2(g) Group VIA Elements (ns2np4) These elements show transition from nonmetal (oxygen, O, sulfur, S, and selenium, Se) to metalloid (tellurium, Te) to metal (polonium, Po). Oxygen occurs as a colorless, odorless gas with O2 molecules. It also has an allotrope, ozone, of molecular formula O3. Sulfur is a brittle, yellow solid with molecular formula, S8. It burns in air to produce the gas sulfur dioxide, SO2. and traces of sulfur trioxide, SO3. Both oxides are acidic. Selenium exists in two allotropic forms, red and gray. Red selenium is a solid composed of Se8 molecules. Gray selenium is a brittle solid that becomes electrically conductive with light shines on it (It has been used to construct devices for measuring light intensity). Selenium forms the acidic oxides SeO2 and SeO3. Selenium dioxide is a white solid that forms when selenium is burned in air. Tellurium is a white, metallic looking substance; it is brittle and a weak electrical conductor. Polonium is a radioactive metal. Group VIA elements form compound with hydrogen of the form H2R; the best know is water, H2O. Hydrogen sulfide or hydrosulfuric acid, H2S, is a poisonous gas with the odor of rotten eggs. Group VIIA Elements, the Halogens (ns2np5) The halogens are reactive nonmetals with the general molecular formula X2, where X symbolizes a halogen. Fluorine, F2, is a pale yellow gas; chlorine, Cl2, a pale greenish-yellow gas; bromine, Br2 , a reddish-brown liquid; and iodine, I2, a bluish-black solid that gives off violet vapor. Little is known about astatine, At, because it is a synthetic elements and is radioactive. It is expected be more metallic than iodine and is perhaps a metalloid. Each halogen forms several compounds with oxygen, but these are unstable. The halogens form compounds with hydrogen with the general formula HX. Hydrogen fluoride is a liquid (B.P. 20∘C); the other binary compounds with hydrogen, called hydrogen hallides, are gases. All are corrosive and dissolve in water to give solutions known generally as hydrohalic acids. For example, hydrogen chloride (HCl) dissolves in water to give hydrochloric acid. Group VIIIA Elements, the Noble Gases (ns2np6) Group VIIIA elements exist as gases consisting of uncombined atoms such as helium, He. For a long time these elements were thought to be chemically inert, because no compounds were known. In the early 1960's several compounds of xenon were formed. Now compounds are also known for krypton and radon. However, these elements re knows as noble gases because the their relative unreactivity. They are also called rare gases or inert gases. Homework: Book questions pg. 324 questions 85, 87, ,89, 90, 92, 95, 97 Practice exercises 7.1-7.7