* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download Unit Analysis Matter Classification
Survey
Document related concepts
Transcript
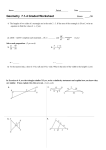
Exam 1 (75 points)—Version A Summer 2009 Chem 161 July 6, 2009 Good luck! Name___________________________ Circle one: Section A (AM lab) Section B (PM lab) 1. Definitions/Terms (10 pts): Choose the BEST answer for each. Only one letter choice is accepted for each term, and no letter should be used more than once. A. Describes how close a measurement is to an accepted value. _____ ionic compound B. Describes how close a series of measurements are to each other, or the fineness of a measurement. _____ Gold Foil experiment C. Is composed of a cation and anion. _____ density D. Does not conduct electricity very well when dissolved in water. _____ evaporation E. Example of a chemical change. _____element F. Example of a physical change. G. Is a ratio of mass to volume. _____mass number H. Proved the atom contains electrons. I. Proved the atom contains a nucleus. _____significant figures J. A substance that cannot be simplied further. _____heterogeneous mixture K. A pure substance. L. Sand and water is an example _____ isotope M. Salt water is an example N. The mass of an atom or ion. _____precision O. The number of protons and neutrons in an isotope. P. Forms of an element that differ in the number of neutrons. Q. Allows the precision of a recorded measurement to be implied. Page 1 of 4 2. True/False (4 pts) a) Rutherford's scattering experiment demonstrated that the mass of an atom was uniformly distributed throughout its volume. True or false? b) Sodium, as an element, is a metal. True or false? c) The answer to (11.98 – 1.995) will have four significant figures. True or false? d) The ancient Greeks in 400 B.C. were able to prove the existence of atoms. True or false? 3. Fill in the blanks. (8 pts). For all symbols, provide the superscripts, as shown for the first one. Symbol # of protons # of neutrons # of electrons 37 Cl-1 Ca 62 20 35 28 provide superscripts 30 4. The effect of alpha particles was studied by Rutherford. What observation from the gold foil experiment led to the idea that the nucleus was very small in volume? (4 pts) 5. In the boxes below, filled circles represent the atoms of one element, while the hollow circles represent atoms of a second element. Provide the best answer for each question below. (8 pt) a. A physical change is best represented by the transformation of box X into box ______ b. A chemical change is best represented by the transformation of box X into box _____ c. True or false: Box b represents a mixture ______ d. What is the likely physical state (solid, liquid or gas) of box X? _______ Explain. Page 2 of 4 6. Nomenclature (16 pts) Give the chemical formula for the following. Include (g) or (aq) when appropriate. Give the name to the following. Only use Roman numerals when appropriate. a) zinc perchlorate i) Ca(CH3COO)2 b) nitrogen trifluoride j) AgNO3 c) sulfuric acid k) NH3 d) hydrogen iodide l) HNO3(aq) e) iron (III) hydroxide m) P4O6 f) chromium (VI) carbonate n) MgCl2 g) calcium nitride o) CuSO3 h) magnesium dihydrogen phosphate p) HCl (aq) 7. Circle the numbers above (1 through 16) for compounds, when dissolved in water, should conduct electricity. (5 pts) 8. A buret is a glassware that has graduations every 0.1 mL. How many decimal places should be recorded for measurements made from this buret? (4 pts) 9. The natural abundance of 81Br is 49.31%. What is the atomic weight of the only other natural isotope of bromine? Use at least four significant figures. (6 pts) Show your work for full credit! Page 3 of 4 10) Dimensional analysis and conversion factors (10 pts) a) The cube pictured below has sides with a length of 0.316522 nanometers (nm). What is the volume of the cube in units of cubic centimeters (cm)? b) Solid tungsten metal (W) is made up of repeating unit cells that are cubes with edges of length 0.316522 nanometers. Each cube contains two tungsten atoms. Tungsten has a density of 19.300 g/cm3 . 1 mole of tungsten is 183.85 grams. Calculate the number of tungsten atoms in one mole of tungsten. Note: You do not need to know what a mole is to solve the problem! Only problem-solving skills! Use as many significant figures as allowed with the given information. Show all work for full credit. If possible, demonstrate your understanding of dimensional analysis as a problem-solving tool. A unit cell containing two W atoms. Page 4 of 4