* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download I. Energy
Survey
Document related concepts
Cell-penetrating peptide wikipedia , lookup
Photosynthetic reaction centre wikipedia , lookup
Cell membrane wikipedia , lookup
Oxidative phosphorylation wikipedia , lookup
Evolution of metal ions in biological systems wikipedia , lookup
Biochemistry wikipedia , lookup
Transcript
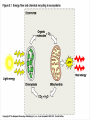
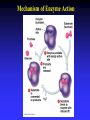
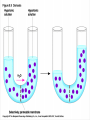
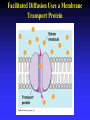
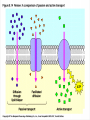
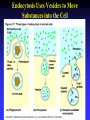
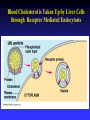
Chapter 5 The Working Cell: Chemical Energetics and Enzymes I. Energy: The capacity to do work. The ability to change matter Can exist in two forms: 1. Kinetic energy: Energy of motion. Energy that is actively performing work. Examples: Heat: Energy of particles in motion. Light: Energy of photons of light 2. Potential energy: Stored energy due to position or arrangement of matter. Examples: Chemical energy: Potential energy of molecules due to the arrangement of atoms. The most important type of energy for living organisms. Position: Bicycle at the top of a hill. Kinds of Energy Chemical Nuclear Electromagnetic Light Electrical Mechanical Heat Sound II. Energy Transformation Energy can be converted from one kind to another. Transformations are inefficient, generating heat. Examples: Light energy -------- --> Chemical energy (sugar) + Heat Chemical energy -- ---> Mechanical energy + Heat Electrical energy --- --> Light energy + Heat Chemical energy --- --> Biological work + Heat Heat is easily measured energy, because all other forms of energy can be converted to heat. From a biological standpoint, heat is a poor kind of energy which is not very useful to do work. Why? Because heat is lost to the environment. III. All energy transformations are subject to the First and Second Laws of Thermodynamics 1. First Law of Thermodynamics: Energy can be transformed (e.g.: chemical to mechanical), but cannot be created nor destroyed. The total amount of energy in the universe is constant. Biological Consequence: Living organisms cannot create the energy they need to live. They must capture it from their environment. Sources of energy used by living organisms: Sun and chemical energy. 2. Second Law of Thermodynamics ENERGY CONVERSIONS ARE INEFFICIENT In any energy transformation, a certain amount of energy is lost as heat. By comparison, living organisms are relatively efficient. Electrical Energy -------> Chemical Energy --> (Gasoline) Chemical Energy --------> (Glucose) ---------> ----------------> 5% Light + 95% Heat 25% Mechanical 75% Heat -----------> 40% ATP + 60% Heat III. Laws of Thermodynamics (Cont.) 2. Second Law of Thermodynamics: The universe inevitably tends toward a state of increased disorder or chaos (entropy). For this reason, energy transformations are inefficient. Entropy (S): Measure of disorder. Disorganized, less usable energy (heat). Only way to overcome entropy, is to put energy into the system. Biological Consequences: Living organisms must constantly take in energy to avoid entropy (disintegration, death and decay). High quality energy is a limited resource, because usable energy, decreases over time. IV. Chemical Reactions Either Store or Release Energy: I. Exergonic Reactions: Release free energy. Also exothermic (release heat). Products have less energy than the reactants. Example: Cellular respiration is an exergonic process: C6H12O6 + 6 O2 ----> 6 CO2 + 6 H2O + Energy Sugar Oxygen High Energy Reactants Carbon Water Dioxide Low Energy Products IV. Chemical Reactions Store or Release Energy: II. Endergonic Reactions: Require net input of free energy. Also endothermic (absorb heat). Products have more energy than the reactants. Create products that are rich in potential energy. Example: Photosynthesis is an endergonic process: 6 CO2 + 6 H2O + Sunlight ----> C6H12O6 + 6 O2 Carbon Water Energy Dioxide Low Energy Reactants Sugar Oxygen High Energy Products Chemical Reactions Either Store or Release Energy Endergonic Reactions Exergonic Reactions Require Energy Higher Energy Products Release Energy Lower Energy Products Metabolism: All chemical processes that occur within a living organism. Either catabolic or anabolic reactions. I. Catabolic Reactions: Release energy (exergonic). Break down large molecules (proteins, polysaccharides) into their building blocks (amino acids, simple sugars). Often coupled to the endergonic synthesis of ATP. Examples: 1. Cellular respiration is a catabolic process: C6H12O6 + 6 O2 -------> 6 CO2 + 6 H2O + Energy Sugar Oxygen Carbon dioxide Water 2. The digestion of sucrose is a catabolic process: Sucrose + Water -------> Glucose + Fructose + Energy Disaccharide Monosaccharides Metabolism: Catabolism + Anabolism II. Anabolic Reactions: Require energy (endergonic). Build large molecules (proteins, polysaccharides) from their building blocks (amino acids, simple sugars). Often coupled to the exergonic breakdown or hydrolysis of ATP. Examples: 1. Photosynthesis is an anabolic process: 6 CO2 + 6 H2O + Sunlight ----> C6H12O6 + 6 O2 Carbon Dioxide Water Sugar Oxygen 2. Synthesis of sucrose is an anabolic process: Glucose + Fructose + Energy -------> Sucrose + H2O Monosaccharides Disaccharide V. ATP: Shuttles Chemical Energy in the Cell Coupled Reactions: Endergonic and exergonic reactions are often coupled to each other in living organisms. The energy released by exergonic reactions is used to fuel endergonic reactions. ATP “shuttles” energy around the cell from exergonic reactions to endergonic reactions. One cell makes and hydrolyzes about 10 million ATPs/second. Cells contain a small supply of ATP molecules (1-5 seconds). ATP powers nearly all forms of cellular work: 1. Mechanical work: Muscle contraction, beating of flagella and cilia, cell movement, movement of organelles, cell division. 2. Transport work: Moving things in & out of cells. 3. Chemical work: All endergonic reactions. A. Structure of ATP (Adenosine triphosphate) Adenine: Nitrogenous base. Ribose: Pentose sugar, same ribose of RNA. Three Phosphate groups: High energy bonds. B. ATP Releases Energy When Phosphates Are Removed: Phosphate bonds are rich in chemical energy and easily broken by hydrolysis: ATP + H2O ----> ADP + Energy + Pi ADP + H2O ----> AMP + Energy + Pi Structure and Hydrolysis of ATP C. Regeneration of ATP: ATP can be regenerated through dehydration synthesis: ADP + Energy + Pi ----> ATP + H2O Phosphorylation: Transfer of a phosphate group to a molecule. Requires energy. The energy required for this endergonic reaction is obtained by trapping energy released by other exergonic reactions (E.g.: Cellular respiration). ATP Shuttles Energy From Exergonic Reactions to Endergonic Reactions VI. Enzymes: Protein molecules that catalyze the reactions of living organisms. Enzymes increase the rate of a chemical reaction without being consumed in the process. Name: Substrate (or activity) + ase suffix Examples: Sucrase Lipase Proteinase Dehydrogenase (Removes H atoms) Enzymes are specific: Catalyze one or a few related reactions. Enzymes are efficient. Can increase the rate of a reaction 10 to billions of times!!!! VI. Enzymes: Enzymes increase the rate of a chemical reaction by lowering the activation energy required to initiate the reaction. Activation energy of a reaction: Energetic barrier that reactant molecules must overcome for reaction to proceed. Creation of new bonds requires breaking of old bonds. Both exergonic and endergonic reactions Transition state :“Intermediate” state of reactants Enzymes Lower the Energy of Activation of a Chemical Reaction Enzyme Mechanism of Action: 1. Binding: Enzyme binds to the reactant(s), forming an enzyme-substrate complex. Substrate: The reactant the enzyme acts upon to lower the activation energy of the reaction. Active site: Region on enzyme where binding to substrate occurs. • Active site dependent upon proper 3-D conformation. Enzyme Mechanism of Action: 2. Induced fit model: After enzyme binds to substrate, it changes shape and lowers activation energy of the reaction by one of several mechanisms: Straining chemical bonds of the substrate Bringing two or more reactants close together Providing “micro-environment” conducive to reaction 3. Release: Once product is made, it is released from active site of enzyme. Enzyme is ready to bind to another substrate molecule. Mechanism of Enzyme Action VII. Environmental Factors Affect Enzyme Function A. Temperature: Optimal temperature. Most reactions are too slow at low temperatures. Most enzymes are denatured over 50-60oC. B. Salt concentration: Optimal concentrations vary for each enzyme. C. pH: Optimal pH varies, but most enzymes work best close to pH 7. Pepsin optimal pH is 2 Amylase optimal pH is 8.5 D. Other essential molecules are required by some enzymes: Cofactors: Inorganic atoms that must bind (Cu, Fe, Zn) Coenzymes: Organic compounds that must bind (vitamins) VII. Regulation of enzyme activity A. Concentration of substrate (enzyme constant) B. Concentration of enzyme (substrate constant) C. Enzyme Inhibitors Competitive inhibitors: Bind to active site of enzyme. Inhibitor resembles normal substrate. Example: • AZT: An AIDS drug. Inhibits an HIV enzyme responsible for viral replication. Resembles nucleotide. Noncompetitive inhibitors: Bind to another site (allosteric site) of enzyme. Active site is altered and unable to bind substrate. Inhibitor usually does not resemble substrate. Competitive versus Noncompetitive Enzyme Inhibition VII. Regulation of enzyme activity D. Feedback Inhibition: Final product of a metabolic pathway can inhibit enzymes that catalyze its synthesis. Enzyme 1 Enzyme 2 Enzyme 3 Enzyme 4 A ---------> B ---------> C ----------> D --------> E (Product) Product (E) inhibits enzyme 1, shutting down pathway. Example: ATP regulates its own production this way. VII. Regulation of enzyme activity E. Inhibition can be temporary or permanent: Reversible inhibition: Inhibitor binds through weak bonds (H-bonds). Inhibitor is released when more substrate is added. Once inhibitor is released, enzyme is functional. Example: Many reactions of cellular metabolism. • ATP feedback inhibition is temporary Irreversible inhibition: Covalent bonds form between enzyme and inhibitor. Enzyme is permanently inactivated. Example: Many drugs, pesticides, and poisons. • Penicillin inhibits bacterial cell wall synthesis. • Malathion inhibits nervous system enzyme (acetylcholinesterase). The Cell Membrane and Cell Transport Functions of Cell Membranes 1. Separate cell from nonliving environment. Form most organelles and partition cell into discrete compartments. 2. Regulate passage of materials in and out of the cell and organelles. Membrane is selectively permeable. 3. Receive information that permits cell to sense and respond to environmental changes. Hormones Growth factors Neurotransmitters 4. Communication with other cells and the organism as a whole. Surface proteins allow cells to recognize each other, adhere, and exchange materials. I. Fluid Mosaic Model of the Membrane 1. Phospholipid bilayer: Major component is a phospholipid bilayer. Hydrophobic tails face inward Hydrophilic heads face water 2. Mosaic of proteins: Proteins “float” in the phospholipid bilayer. 3. Cholesterol: Maintains proper membrane fluidity. The outer and inner membrane surfaces are different. Membrane Phospholipids Form a Bilayer The Membrane is a Fluid Mosaic of Phospholipids and Proteins Notice that inner and outer surfaces are different A. Fluid Quality of Plasma Membranes In a living cell, membrane has same fluidity as salad oil. Unsaturated hydrocarbon tails INCREASE membrane fluidity Phospholipids and proteins drift laterally. Phospholipids move very rapidly Proteins drift in membrane more slowly Cholesterol: Alters fluidity of the membrane Decreases fluidity at warmer temperatures (> 37oC) Increases fluidity at lower temperatures (< 37oC) B. Membranes Contain Two Types of Proteins 1. Integral membrane proteins: Inserted into the membrane. Hydrophobic region is adjacent to hydrocarbon tails. 2. Peripheral membrane proteins: Attached to either the inner or outer membrane surface. Functions of Membrane Proteins: 1. Transport of materials across membrane 2. Enzymes 3. Receptors of chemical messengers 4. Identification: Cell-cell recognition 5. Attachment: Membrane to cytoskeleton Intercellular junctions Membrane Proteins Have Diverse Functions C. Membrane Carbohydrates and Cell-Cell Recognition Found on outside surface of membrane. Important for Cell-cell recognition: Ability of one cell to “recognize” other cells. Allows immune system to recognize self/non-self Include: • Glycolipids: Lipids with sugars • Glycoproteins: Proteins with sugars • Major histocompatibility proteins (MHC or transplantation antigens). Vary greatly among individuals and species. Organ transplants require matching of cell markers and/or immune suppression. The cell plasma membrane is Selectively Permeable A. Permeability of the Lipid Bilayer 1. Non-polar (Hydrophobic) Molecules • Dissolve into the membrane and cross with ease • The smaller the molecule, the easier it can cross • Examples: O2 , hydrocarbons, steroids 2. Polar (Hydrophilic) Molecules • Small polar uncharged molecules can pass through easily (e.g.: H2O , CO2) • Large polar uncharged molecules pass with difficulty (e.g.: glucose) 3. Ionic (Hydrophilic) Molecules • Charged ions or particles cannot get through (e.g.: ions such as Na+ , K+ , Cl- ) Transport Proteins in the membrane: Integral membrane proteins that allow for the transport of specific molecules across the phospholipid bilayer of the plasma membrane. How do they work? May provide a “hydrophilic tunnel” (channel) May bind to molecule and physically move it Are specific for the atom/molecule transported III. Passive transport: Diffusion of molecules across the plasma membrane A. Diffusion: The net movement of a substance from an area of high concentration to area of low concentration. Does not require energy. B. Passive transport: The diffusion of substance across a biological membrane. Only substances which can cross bilayer by themselves or with the aid of a protein Does not require the cell’s energy Passive Transport: Diffusion Across a Membrane Does Not Require Energy IV. Osmosis: The diffusion of water across a semi-permeable membrane. Through osmosis water will move from an area with higher water concentration to an area with lower water concentration. Solutes can’t move across the semi-permeable membrane. Osmotic Pressure: Ability of a solution to take up water through osmosis. Example: The cytoplasm of a cell has a certain osmotic pressure caused by the solutes it contains. There are three different types of solution when compared to the interior (cytoplasm) of a cell: 1. Hypertonic solution: Higher osmotic pressure than cell due to: Higher solute concentration than cell or Lower water concentration than cell. 2. Hypotonic solution: Lower osmotic pressure than cell due to: Lower solute concentration than cell or Higher water concentration than cell. 3. Isotonic solution: Same osmotic pressure than cell. Equal concentration of solute(s) and water than cell. V. Cells depend on proper water balance Animal Cells: Do best in isotonic solutions. Examples: 0.9% NaCl (Saline) 5% Glucose If solution is not isotonic, cell will be affected: Hypertonic solution: Cell undergoes crenation. Cell “shrivels” or shrinks. Example: 5% NaCl or 10% glucose Hypotonic solution: Cell undegoes lysis. Cell swells and eventually bursts. Example: Pure water. V. Cells depend on proper water balance Plant Cells: Do best in hypotonic solutions, because the cell wall protects from excessive uptake of water. Hypertonic solution: Cell undergoes plasmolysis. Cell membrane shrivels inside cell wall. Isotonic solution: Cell becomes flaccid or wilts. Hypotonic solution: Turgor. Increased firmness of cells due to osmotic pressure. This is the reason why supermarkets spray fruits and vegetables with pure water, making them look firm and fresh. VI. Facilitated Diffusion: Some substances cannot cross the membrane by themselves due to their size or charge. Membrane proteins facilitate the transport of solutes down their concentration gradient. No cell energy is required. Transport Proteins Specific : Only transport very specific molecules (binding site) Glucose Specific ions (Na+, K+, Cl- ) Facilitated Diffusion Uses a Membrane Transport Protein VI. Active Transport: Proteins use energy from ATP to actively “pump” solutes across the membrane Solutes are moved against a concentration gradient. Energy is required. Example: The Na+-K+ ATPase pump: Energy of ATP hydrolysis is used to move Na+ out of the cell and K+ into the cell Endocytosis: Moving materials into cell with vesicles. Requires use of cell energy. 1. Pinocytosis (“Cell drinking”): Small droplets of liquid are taken into the cell through tiny vesicles. Not a specific process, all solutes in droplets are taken in. 2. Phagocytosis (“Cell eating”): Large solid particles are taken in by cell. Example: Amoebas take in food particles by surrounding them with cytoplasmic extensions called pseudopods. Particles are surrounded by a vacuole. Vacuole later fuses with the lysosome and contents are digested. Endocytosis Uses Vesicles to Move Substances into the Cell Endocytosis: 3. Receptor mediated endocytosis: Highly specific. Materials moved into cell must bind to specific receptors first. Example: Low density lipoproteins (LDL): Main form of cholesterol in blood. Globule of cholesterol surrounded by single layer of phospholipids with embedded proteins. Liver cell receptors bind to LDL proteins and remove LDLs from blood through receptor mediated endocytosis. Familial hypercholesterolemia: Genetic disorder in which gene for the LDL receptor is mutated. Disorder found in 1 in 500 human babies worldwide. Results in unusually high levels of blood cholesterol. Blood Cholesterol is Taken Up by Liver Cells through Receptor Mediated Endocytosis Exocytosis: Used to export materials out of cell. Materials in vesicles fuse with cell membrane and are released to outside. Tear glands export salty solution. Pancreas uses exocytosis to secrete insulin.