* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download Loss of Starch Granule Initiation Has a
Plant tolerance to herbivory wikipedia , lookup
Evolutionary history of plants wikipedia , lookup
Plant secondary metabolism wikipedia , lookup
Venus flytrap wikipedia , lookup
History of herbalism wikipedia , lookup
Arabidopsis thaliana wikipedia , lookup
Photosynthesis wikipedia , lookup
Plant breeding wikipedia , lookup
Flowering plant wikipedia , lookup
Plant defense against herbivory wikipedia , lookup
Ornamental bulbous plant wikipedia , lookup
Plant stress measurement wikipedia , lookup
Plant nutrition wikipedia , lookup
History of botany wikipedia , lookup
Plant morphology wikipedia , lookup
Historia Plantarum (Theophrastus) wikipedia , lookup
Plant use of endophytic fungi in defense wikipedia , lookup
Plant physiology wikipedia , lookup
Plant reproduction wikipedia , lookup
Plant evolutionary developmental biology wikipedia , lookup
Plant ecology wikipedia , lookup
Perovskia atriplicifolia wikipedia , lookup
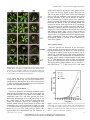
Loss of Starch Granule Initiation Has a Deleterious Effect on the Growth of Arabidopsis Plants Due to an Accumulation of ADP-Glucose1[W] Paula Ragel, Sebastian Streb, Regina Feil, Mariam Sahrawy, Maria Grazia Annunziata, John E. Lunn, Samuel Zeeman, and Ángel Mérida* Instituto de Bioquímica Vegetal y Fotosíntesis, Consejo Superior de Investigaciones Científicas-University of Sevilla, 41092 Seville, Spain (P.R., A.M.); Department of Biology, Eidgenössische Technische Hochschule Zürich, CH–8092 Zurich, Switzerland (S.S., S.Z.); Estación Experimental del Zaidín, Consejo Superior de Investigaciones Científicas, 18008 Granada, Spain (M.S.); and Max Planck Institute of Molecular Plant Physiology, D–14476 Potsdam-Golm, Germany (R.F., M.G.A., J.E.L.) STARCH SYNTHASE4 (SS4) is required for proper starch granule initiation in Arabidopsis (Arabidopsis thaliana), although SS3 can partially replace its function. Unlike other starch-deficient mutants, ss4 and ss3/ss4 mutants grow poorly even under longday conditions. They have less chlorophyll and carotenoids than the wild type and lower maximal rates of photosynthesis. There is evidence of photooxidative damage of the photosynthetic apparatus in the mutants from chlorophyll a fluorescence parameters and their high levels of malondialdehyde. Metabolite profiling revealed that ss3/ss4 accumulates over 170 times more ADP-glucose (Glc) than wild-type plants. Restricting ADP-Glc synthesis, by introducing mutations in the plastidial phosphoglucomutase (pgm1) or the small subunit of ADP-Glc pyrophosphorylase (aps1), largely restored photosynthetic capacity and growth in pgm1/ss3/ss4 and aps1/ss3/ss4 triple mutants. It is proposed that the accumulation of ADP-Glc in the ss3/ss4 mutant sequesters a large part of the plastidial pools of adenine nucleotides, which limits photophosphorylation, leading to photooxidative stress, causing the chlorotic and stunted growth phenotypes of the plants. The metabolism of starch plays an essential role in the physiology of plants. Starch breakdown provides the plant with carbon skeletons and energy when the photosynthetic machinery is inactive (transitory starch) or in the processes of germination and sprouting (storage starch). Deficiencies in the accumulation of transitory starch in Arabidopsis (Arabidopsis thaliana) have been described previously, specifically in mutants affected in the plastidial phosphoglucomutase (PGM1) or the small subunit (APS1) of the ADP-Glc pyrophosphorylase (AGPase). While they are described as “starchless,” they actually contain small amounts of starch (1%–2% of the wild-type levels; Streb et al., 2009) and share similar phenotypic alterations, such as growth retardation when cultivated under a short-day photoregime and increased levels of soluble sugars 1 This work was supported by the Comisión Interministerial de Ciencia y Tecnología and the European Union-FEDER (grant no. BIO2009–07040), by the Junta de Andalucía (grant nos. P07–CVI– 02795 and P09–CVI–4704), and by the Spanish Ministry of Education (Formación de Personal Universitario grant to P.R.). * Address correspondence to [email protected]. The author responsible for distribution of materials integral to the findings presented in this article in accordance with the policy described in the Instructions for Authors (www.plantphysiol.org) is: Ángel Mérida ([email protected]). [W] The online version of this article contains Web-only data. www.plantphysiol.org/cgi/doi/10.1104/pp.113.223420 during the light phase and reduced levels during the night (Caspar et al., 1985; Lin et al., 1988b; Schulze et al., 1991). Carbon partitioning is altered in these plants. As photosynthate cannot be accumulated as starch, it is diverted via hexose phosphates in the cytosol to the synthesis of Suc, which accumulates together with the hexose sugars, Glc and Fru (Caspar et al., 1985). In Arabidopsis, there are five starch synthase isoforms: one granule-bound starch synthase and four soluble starch synthases: SS1, SS2, SS3, and SS4. We have described previously an Arabidopsis mutant plant lacking SS3 and SS4 that is also severely affected in the accumulation of starch (Szydlowski et al., 2009). SS4 is involved in the initiation of the starch granule and controls the number of granules per chloroplast (Roldán et al., 2007). The elimination of SS3 in an ss4 background leads to an absence of starch in most of the chloroplasts, despite the fact that SS1 and SS2 are still present and total starch synthase activity is only reduced by 35% (Szydlowski et al., 2009). However, a very small proportion of chloroplasts of this mutant plant contain a single huge starch granule, which is also a characteristic of chloroplasts in the ss4 single mutant (D’Hulst and Mérida, 2012). Thus, like aps1 and pgm1, ss3/ss4 plants contain only small amounts of starch. However, unlike aps1 or pgm1 plants, most of the cells of this mutant have empty chloroplasts, without starch (Szydlowski et al., 2009). Plant PhysiologyÒ, September 2013, Vol. 163, pp. 75–85, www.plantphysiol.org Ó 2013 American Society of Plant Biologists. All Rights Reserved. Downloaded from on June 18, 2017 - Published by www.plantphysiol.org Copyright © 2013 American Society of Plant Biologists. All rights reserved. 75 Ragel et al. In this work, we have analyzed the phenotypic effects of the impaired starch accumulation of ss3/ss4 plants. We show that this mutant displays phenotypic changes that are not found in other mutants with very low levels of starch, such as aps1 or pgm1 plants. We provide evidence that extremely high levels of ADP-Glc accumulate in the ss3/ss4 plants. Using reverse genetics to block the pathway of starch synthesis upstream of the starch synthases reduced the level of ADP-Glc in ss3/ss4 plants and reverted the other phenotypic traits. This suggests that ADPGlc accumulation is the causal factor behind the chlorotic and stunted growth phenotypes of the ss3/ss4 mutant. RESULTS Phenotypic Characterization of an ss3/ss4 Double Mutant Arabidopsis plants mutated in both SS4 and SS3 genes accumulate very low levels of starch in leaves (around 12% of wild-type values; Fig. 1A) but have slightly higher levels of soluble sugars, particularly Suc (Fig. 1, B–D). However, this double mutant plant displays some phenotypic effects, such as poor growth rate under a long-day photoregime and pale green color (Fig. 2), that are not observed in other starch synthase-deficient mutants (Delvallé et al., 2005; Zhang et al., 2005) or in mutants that accumulate very low amounts of starch, such as those affected in APS1 (Lin Figure 1. Starch, sugar, and adenine nucleotide contents of wild-type and mutant Arabidopsis rosettes. Wild type (Col-0), ss3, ss4, ss3/ss4, aps1, pgm1, aps1/ss3/ss4, and pgm1/ss3/ss4 plants were grown under LD conditions (16 h of light, 20˚C/8 h of dark, 18˚C) and an irradiance of 150 mE m22 s21. Plants were harvested at midday at the nineleaf stage and processed as described in “Materials and Methods.” Eight to 10 plants per sample were used for the ss3/ss4 mutant and four to five plants for the other genotypes. Values represent means 6 SD of determinations on six independent samples (three independent samples for measurements of starch, sugars, and ADP-Glc in ss3s/ ss4). Letters indicate samples that were not significantly different (P $ 0.05) according to one-way ANOVA (HolmSidak test). There were no significant differences in AMP content between the eight genotypes. ADPG, ADP-Glc; FW, fresh weight. 76 Plant Physiol. Vol. 163, 2013 Downloaded from on June 18, 2017 - Published by www.plantphysiol.org Copyright © 2013 American Society of Plant Biologists. All rights reserved. Growth Defects of ss3/ss4 Linked to High ADP-Glucose starch and showed essentially the same growth rate as aps1 (data not shown). Figure 2 shows that aps1, ss4, and ss3/ss4 plants all grew more slowly than wild-type plants in the SD condition, with growth being most severely restricted in the ss3/ss4 mutant. In contrast, the ss3 mutant grew slightly faster than wild-type plants. Increasing the length of the light period greatly increased the growth of the aps1 mutant, which was restored to nearly wild-type levels in LL conditions (Fig. 2). However, the growth of ss3/ss4 in LD and LL conditions remained severely limited, while the phenotype of ss4 was intermediate between that of ss3/ss4 and wild-type plants (Figs. 2 and 3). These results indicate that the poor growth of ss3/ss4, and to a lesser extent of ss4, is not caused solely by the impairment in starch accumulation and that some other metabolic perturbation, which is specific to these mutants, restricts their growth. Photosynthetic Activity The low growth rate observed in ss4 and ss3/ss4 plants suggests that their photosynthetic activity is negatively affected by the defects in starch synthesis. To investigate which aspects of photosynthesis are affected, CO2 assimilation rate versus photosynthetically active radiation were determined on attached leaves of plants grown under a LD photoregime at 130 mmol m22 s21 and ambient CO2 using an open gas-exchange system. The light-response curves are shown in Figure 4. The light-saturated photosynthesis rates of ss4 (6.87 6 0.39 mmol CO2 m22 s21) and ss3/ss4 (2.51 6 0.52 mmol Figure 2. Effects of photoperiod on growth phenotypes of starch-deficient mutants. Plants were grown in growth cabinets under different photoperiods: LD (16 h of light/8 h of dark), SD (8 h of light/16 h of dark), and LL. The growth of the different mutants was documented by photographs of representative 3-week-old plants. WT, Wild type. et al., 1988a; Fig. 1A) or in the chloroplastic PGM1 (Caspar et al., 1985; Fig. 1A). We have carried out a comprehensive phenotypic analysis of the ss3/ss4 mutant in order to identify the metabolic cause of its distinctive growth and morphological traits. Growth of the ss3/ss4 Mutant Plants were cultivated in controlled conditions under different photoregimes: long day (LD; 16 h of light/8 h of dark), short day (SD; 8 h of light/16 h of dark), and continuous light (LL), and their growth was documented by photographs of 21-d-old plants (Fig. 2) and by a time course of the growth of plants cultivated under LD conditions (Fig. 3). The Arabidopsis aps1 mutant, which lacks the small subunit of AGPase and accumulates only very low amounts of starch, was used as a control, and its growth rate was compared with those of ss3, ss4, ss3/ss4, and wild-type plants. The pgm1 mutant also accumulated very low amounts of Figure 3. Growth rates of wild-type (WT) Col-0, ss3, ss4, and ss3/ss4 plants. Plants were grown in growth cabinets under a LD photoperiod (16 h of light/8 h of dark). Aerial parts of the plants (five plants per sample) were weighed on the days indicated. Each point represents the mean 6 SD of three independent experiments. Plant Physiol. Vol. 163, 2013 77 Downloaded from on June 18, 2017 - Published by www.plantphysiol.org Copyright © 2013 American Society of Plant Biologists. All rights reserved. Ragel et al. antenna and accessory pigments (chlorophylls a and b and carotenoids) were about 45% lower in the double mutant than in wild-type plants. There was a smaller decrease, to around 63% of the wild type, in the ss4 single mutant. While chlorophyll and carotenoid levels were decreased, the ss4 and ss3/ss4 mutants accumulated more anthocyanins than the controls, with 1.4- and 5.8-fold higher levels than wild-type plants, respectively (Fig. 5). The pigment content of the ss3 mutant was not significantly different from the wild type. Photooxidative Stress Figure 4. Photosynthetic assimilation rates. Light-response curves of CO2 assimilation rates (A) of wild-type (WT), ss3, ss4, and ss3/ss4 plants were measured in a leaf chamber. Three-week-old plants grown in growth cabinets under a LD photoperiod were used to determine the CO2 assimilation rates. Each point represents the mean 6 SD of five independent determinations. CO2 m22 s21) in these conditions were substantially lower (65% and 24% of the control rate, respectively) than the maximum value obtained for wild-type plants (10.57 6 0.64 mmol CO2 m22 s21). Values obtained for the ss3 mutant plants (10.63 6 0.32 mmol CO2 m22 s21) were not significantly different from wild-type plants (Fig. 4). Measurements of modulated chlorophyll a fluorescence (see “Materials and Methods”) indicated that the maximum quantum efficiency of PSII photochemistry (Fv/Fm) was drastically reduced in the ss3/ss4 mutant (0.63 6 0.040 and 0.82 6 0.002 for ss3/ss4 and wild-type plants, respectively; Table I). A reduction was also observed in the PSII operating efficiency (FPSII), indicating a decrease in the relative quantum yield of linear electron transfer through the photosystems (Baker, 2008). The values of the parameters obtained for ss4 mutant plants were also significantly different from those obtained for wild-type plants (Table I), being intermediate between wild-type and ss3/ss4 double mutant plants. This suggests that the single ss4 mutation leads to negative effects on both PSII efficiency and the CO2 assimilation rate of the plant, which are exacerbated by loss of the SS3 gene function. Reducing the levels of antenna pigments would be expected to help balance the input and utilization of light energy in the ss4 and ss3/ss4 mutants. Nevertheless, despite this adaptive response, blockage of the electron flux between photosystems could result in photooxidative damage in these plants. Photooxidative damage caused by reactive oxygen species (ROS) in ss4 and ss3/ss4 was confirmed by their higher content of malondialdehyde (MAD; Fig. 6), which is a marker of membrane lipid peroxidation caused by elevated levels of ROS (Janero, 1990). The MAD content was 2.5 times higher in ss3/ss4 than in wild-type plants, while ss4 plants had levels intermediate between ss3/ss4 and the wild type, being 1.5 times higher than wild-type plants (Fig. 6). The ss3/ss4 and ss4 Mutants Accumulate ADP-Glc ADP-Glc is the substrate for starch synthesis. Therefore, impairment of starch synthesis as found in ss4 and ss3/ss4 mutants (Roldán et al., 2007; Szydlowski et al., 2009) might be expected to affect the accumulation of ADP-Glc, especially in the ss3/ss4 mutant, which has very low levels of starch. ADP-Glc was measured in wild-type and mutant plants by high-performance anionexchange chromatography coupled to tandem mass spectrometry (MS/MS; Lunn et al., 2006). This method provides greater specificity and sensitivity than the HPLC-based methods with UV detection that have Table I. Chlorophyll a fluorescence parameters of wild-type, ss3, ss4, ss3/ss4, aps1, and aps1/ss3/ss4 plants Pigment Content The lower yield of linear electron flux through the photosystems observed in ss3/ss4 and ss4 mutant plants could trigger some adaptive responses, such as a decrease in the light-harvesting antenna size, in order to prevent possible photooxidative damage (Niyogi, 1999). Such a response would account for the pale green color that is characteristic of ss4 and ss3/ss4 mutants compared with the other starch-deficient mutants. To test this hypothesis, we measured the levels of different pigments in ss3/ss4 plants and compared them with the pigment contents of both the single parental mutants and wild-type plants. Figure 5 shows that the levels of Fv /Fm and FPSII at 130 mE m22 s21 were determined in dark-adapted plants. Values are means 6 SE of four independent determinations. Significance is indicated as follows: a, not significantly different from the wild type according to Student’s t test (P $ 0.01); b, significantly different from the wild type according to Student’s t test (P , 0.01). Genotype Fv /Fm FPSII Wild type (Col-0) ss3 ss4 ss3/ss4 aps1 aps1/ss3/ss4 0.816 6 0.001 0.801 6 0.001 a 0.754 6 0.008 b 0.631 6 0.040 b 0.803 6 0.003 a 0.802 6 0.003 a 0.513 6 0.000 0.483 6 0.008 a 0.350 6 0.006 b 0.220 6 0.044 b 0.480 6 0.010 a 0.478 6 0.027 a 78 Plant Physiol. Vol. 163, 2013 Downloaded from on June 18, 2017 - Published by www.plantphysiol.org Copyright © 2013 American Society of Plant Biologists. All rights reserved. Growth Defects of ss3/ss4 Linked to High ADP-Glucose the plastidial enzymes that synthesize ADP-Glc and Glc-1-P, respectively. The levels of Suc, Fru, and Glc in ss3/ss4 plants were lower than in aps1 or pgm1, which contained 2- to 3-fold higher soluble sugar levels than wild-type plants (Fig. 1, B–D). Measurements of other metabolites by high-performance anion-exchange chromatography coupled to MS/MS showed a 2-fold higher level of trehalose 6-phosphate in ss3/ss4 compared with the wild type (Supplemental Fig. S1), presumably reflecting the 2-fold higher Suc content of the mutant (Fig. 1D). There were no striking differences between ss3/ss4 and the wild type in the levels of other sugar phosphates or glycolytic intermediates, apart from a decrease in Fru-1,6-bisP in the mutant (Supplemental Fig. S1). Citrate, aconitate, and isocitrate were slightly elevated in the mutant, while the other tricarboxylic acid cycle intermediates were either unchanged (2-oxoglutarate and succinate) or slightly lower (malate and fumarate) in the ss3/ss4 mutant (Supplemental Fig. S1). Restricting Plastidial ADP-Glc Synthesis Substantially Restores Growth in the ss3/ss4 Plants The high levels of ADP-Glc found in ss3/ss4 plants are presumably caused by an imbalance between the rates of ADP-Glc synthesis by AGPase and the consumption for starch synthesis, which is essentially blocked in this mutant. As a result, it seems likely that much of the adenine nucleotides in the chloroplasts will be effectively locked away in this large pool of ADP-Glc, restricting photophosphorylation, CO2 Figure 5. Photosynthetic pigments and anthocyanin contents. Plants were grown in growth cabinets under a LD photoperiod, and discs of rosette leaves from four different plants per sample were used to determine the chlorophyll a and b, carotenoid, and anthocyanin contents of wild-type (WT), ss3, ss4, and ss3/ss4 plants (A) and wild-type, aps1, pgm1, ss3/ss4, aps1/ss3/ss4, and pgm1/ss3/ss4 plants (B). Values are means 6 SD of three independent experiments. Asterisks indicate values that are significantly different from the wild type according to Student’s t test (P , 0.05). fw, Fresh weight. generally been used to measure ADP-Glc in plants. The ss3/ss4 plants were found to have extremely high levels of ADP-Glc (341 nmol g21 fresh weight), which was around 170-fold higher than in wild-type plants (Fig. 1E). The content of this compound in other mutants that accumulate only trace amounts of starch, such as aps1 and pgm1, was lower than in wild-type plants (Fig. 1E) as a consequence of the mutations in Figure 6. MAD accumulation. MAD content in leaves of wild-type (WT), ss3, ss4, and ss3/ss4 plants grown under a LD photoperiod was determined using the thiobarbituric acid method. Values are means 6 SD of four independent experiments. Asterisks indicate values that are significantly different from the wild type according to Student’s t test (P , 0.05). Plant Physiol. Vol. 163, 2013 79 Downloaded from on June 18, 2017 - Published by www.plantphysiol.org Copyright © 2013 American Society of Plant Biologists. All rights reserved. Ragel et al. fixation, and other process that are dependent on ATP. To test this hypothesis, we crossed ss3/ss4 plants with mutant plants lacking the small subunit of AGPase (aps1) or the plastidial isoform of PGM (pgm1). Both mutants are greatly impaired in the synthesis of plastidial ADP-Glc and accumulate only very small amounts of starch (Stitt and Zeeman, 2012; Fig. 1A). Progeny of the respective crosses were analyzed to identify aps1/ss3/ss4 and pgm1/ss3/ss4 triple mutants (see “Materials and Methods”), and these plants were selected for subsequent analyses. Mutant plants cultured under a 16-h-light/8-h-dark (aps1/ss3/ss4) or a 12-h-light/12-h-dark (pgm1/ss3/ss4) photoregime showed the same growth rate as the aps1 or pgm1 parental plants, respectively, which were higher than the growth rate of ss3/ss4 parental plants (Fig. 7; Supplemental Fig. S2). Attached leaves of aps1/ss3/ss4 plants or whole pgm1/ss3/ss4 plants grown under the same conditions detailed above were used to determine their CO2 assimilation rates. Under saturating light, the CO2 assimilation rates of the ss3/ss4 (2.51 6 0.52 mmol CO2 m22 s21) and aps1 (6.08 6 0.23 mmol CO2 m22 s21) mutants were 61% and 36% lower, respectively, than in wild-type plants (9.29 6 1.01 mmol CO2 m22 s21; Fig. 8). The CO2 assimilation rate of aps1/ss3/ss4 plants (6.69 6 0.77 mmol CO2 m22 s21) was similar to that found for aps1 plants, indicating that the nearly complete loss of AGPase activity overrides the severe inhibition of photosynthesis in the ss3/ss4 mutant background (Fig. 8). Similarly, restricting ADP-Glc synthesis via the loss of plastidial PGM activity restored photosynthetic rates in the pgm1/ss3/ss4 mutant to wild-type levels (Fig. 9). Measurements of Fv/Fm and FPSII showed that both of these photosynthetic parameters in the aps1/ss3/ss4 and pgm1/ss3/ss4 triple mutants had reverted to wildtype values (Tables I and II). The levels of photosynthetic pigments (chlorophylls a and b and carotenoids) in aps1/ss3/ss4 were similar to those of wild-type plants (Fig. 5), providing further evidence that restricting ADP-Glc counteracted the negative effects of the loss of SS3 and SS4 activity on photosynthesis. Levels of anthocyanins in the aps1/ss3/ss4 mutant plants were much lower than in the parental ss3/ss4 mutant, being slightly higher than in wild-type plants but similar to the parental aps1 mutant (Fig. 5). Metabolite profiling of the triple mutants revealed that they accumulated 87% to 97% less ADP-Glc than the ss3/ss4 mutant (Fig. 1E). However, the levels of ADP-Glc in aps1/ss3/ss4 plants were still considerably higher (around 23-fold) than in wild-type plants but only about 5-fold higher than the wild type in the pgm1/ss3/ss4 mutant. To assess the impact of the massive accumulation of ADP-Glc on other adenine nucleotides, we measured ATP, ADP, and AMP in mutant and wild-type plants. ATP was about 27% lower in the ss3/ss4 mutant than in wild-type plants (Fig. 1F). The difference was statistically significant (P = 0.047) according to Student’s t test but not significant according to one-way ANOVA (Holm-Sidak test; Fig. 1F). ADP also showed a tendency to be decreased in the ss3/ss4 mutant (Fig. 1G). Both Figure 7. Growth phenotypes of wild-type (WT), aps1, pgm1, ss3/ss4, aps1/ss3/ss4, and pgm1/ss3/ss4 plants. The photographs show 3-weekold plants cultivated in growth cabinets under a 16-h-light/8-h-dark (aps1, ss3/ss4, and aps1/ss3/ss4) or a 12-h-light/12-h-dark (pgm1, ss3/ss4, and pgm1/ss3/ss4) photoperiod. ATP and ADP were significantly lower in the pgm mutant than in the wild type (Fig. 1F). The total adenine nucleotide pools (ATP + ADP + AMP + ADP-Glc) in the ss3/ss4 and ss4 mutants were about four times higher than in wild-type plants, with ADP-Glc constituting 81%, 77%, and 2% of the total adenylate pool, respectively (Fig. 1; Supplemental Fig. S3). The ATP, ADP, and total adenylate pool sizes in the aps1/ss3/ss4 80 Plant Physiol. Vol. 163, 2013 Downloaded from on June 18, 2017 - Published by www.plantphysiol.org Copyright © 2013 American Society of Plant Biologists. All rights reserved. Growth Defects of ss3/ss4 Linked to High ADP-Glucose Figure 8. Photosynthetic assimilation rates. Light-response curves of CO2 assimilation rates (A) of wild-type (WT), aps1, ss3/ss4, and aps1/ss3/ss4 plants were measured in a leaf chamber. Three-week-old plants grown in growth cabinets under a LD photoperiod were used to determine the CO2 assimilation rates. Each point represents the mean 6 SD of five independent determinations. and pgm/ss3/ss4 triple mutants were indistinguishable from the wild-type levels (Fig. 1, F and G; Supplemental Fig. S3). DISCUSSION We have previously reported that Arabidopsis requires SS4 or SS3 to synthesize starch (Szydlowski et al., 2009), strongly implicating SS4 and SS3 in the process of granule initiation. In subsequent analyses of the ss3/ss4 mutant, we have observed that a small number of chloroplasts do contain single starch granules with a distinctive shape, being more rounded than the discoid granules usually found in Arabidopsis leaves (D’Hulst and Mérida, 2012). This suggests that in the absence of SS4, stochastic initiation of starch granules can still occur, albeit very infrequently, even when SS3 is also missing (D’Hulst and Mérida, 2012). This would explain the trace amounts of starch in the ss3/ss4 plants (Fig. 1A). The most obvious phenotypic traits of the ss3/ss4 plants are their severely restricted growth and chlorotic leaves (Figs. 2 and 3). Transitory starch reserves provide the plant with a buffer itself against diurnal variation in carbon and energy supplies (Sun et al., 1999; Smith and Stitt, 2007). Mutants impaired in starch synthesis, such as aps1 and pgm1, accumulate high levels of Suc and hexoses (Caspar et al., 1985) as a consequence of their inability to store net photosynthate in starch. However, the total amount of carbohydrate accumulated as soluble sugars in starchdeficient mutants during the day is much less than the amount of starch accumulated by wild-type plants (Fig. 1, A–D). Furthermore, soluble sugars are directly available for export and respiration, so these reserves, which are already smaller than those in wild-type plants, are rapidly depleted during the first hours of the night. This leads to carbon starvation by the end of the night, which suppresses growth by mechanisms that are not yet fully understood but probably involve the inhibition of translation and other biosynthetic processes and wasteful turnover of proteins (Gibon et al., 2004). The carbon starvation diminishes as the length of the night period is reduced; hence, the growth rates of the aps1 (Fig. 2) and pgm1 mutant plants in LD or LL conditions were practically the same as that observed in wild-type control plants (Lin et al., 1988a). The ss3/ss4 plants show a severe dwarf phenotype even in LD or LL photoregimes (Figs. 2 and 3), suggesting that the impairment in starch synthesis on its own is not the underlying cause of the poor growth of these plants and that other metabolic changes must be responsible for their stunted phenotype. In chloroplasts, the substrate for starch synthesis is derived from the Calvin-Benson cycle in the form of Fru-6-P. This is converted to Glc-6-P and then Glc-1-P in two reversible reactions catalyzed by phosphoglucose isomerase and phosphoglucomutase, respectively. The next step in the pathway, the synthesis of ADP-Glc by AGPase, is rendered irreversible by the hydrolysis of the coproduct, pyrophosphate, by inorganic pyrophosphatase (Ballicora et al., 2003). Thus, loss of plastidial phosphoglucose isomerase, PGM, or AGPase activity essentially blocks both ADP-Glc and starch synthesis, although a small amount of starch may be synthesized from imported hexose phosphate or ADP-Glc in young developing leaves, bypassing the metabolic lesions in Figure 9. Photosynthetic assimilation rates. Whole, 3-week-old plants were used to determine the photosynthetic CO2 assimilation rates (A) in a multichamber system at an irradiance of 150 mE m22 s21. Values are means 6 SD of four biological replicates. WT, Wild type. Plant Physiol. Vol. 163, 2013 81 Downloaded from on June 18, 2017 - Published by www.plantphysiol.org Copyright © 2013 American Society of Plant Biologists. All rights reserved. Ragel et al. Table II. Chlorophyll a fluorescence parameters of wild-type, ss3, ss4, ss3/ss4, pgm1, and pgm1/ss3/ss4 plants Fv /Fm and FPSII at 130 mE m22 s21 were determined in dark-adapted plants. Values are means 6 SE of four independent determinations. Significance is indicated as follows: a, not significantly different from the wild type according to Student’s t test (P $ 0.01); b, significantly different from the wild type according to Student’s t test (P , 0.01). Genotype Fv /Fm FPSII Wild type (Col-0) ss3 ss4 ss3/ss4 pgm1 pgm1/ss3/ss4 0.808 6 0.0005 0.803 6 0.0015 a 0.771 6 0.0015 b 0.726 6 0.005 b 0.790 6 0.000 a 0.779 6 0.0005 a 0.394 6 0.009 0.414 6 0.003 a 0.315 6 0.002 b 0.204 6 0.005 b 0.381 6 0.003 a 0.391 6 0.006 a these mutants (Streb et al., 2009; Bahaji et al., 2011). The situation is different in the ss3/ss4 mutant, where starch synthesis is limited not by the availability of ADP-Glc but by the almost complete failure of starch granule initiation and the consequent lack of primers for glucan synthesis. Given the pyrophosphatasedriven irreversibility of the AGPase reaction, there is no obvious reason why ADP-Glc should not continue to be synthesized. Starch synthesis is the main route for catabolizing ADP-Glc in the chloroplasts (Ball et al., 2011), but as this route is blocked in the ss3/ss4 mutant, there is likely to be an imbalance between ADP-Glc production and consumption, leading to its accumulation in the chloroplast stroma. Metabolite profiling confirmed that the ss3/ss4 mutant does indeed accumulate massive amounts of ADP-Glc (341 6 16 nmol g21 fresh weight), reaching levels that are over 170 times higher than in wild-type plants (Fig. 1E). In addition, levels of soluble sugars are only slightly increased in the ss3/ss4 mutant (Fig. 1, B–D), likely caused by the severe decrease of CO2 fixation in this mutant, which determines that the carbon diverted to soluble sugars, such as Suc, as consequence of the blockage of starch synthesis is lower than in aps1 or pgm1 mutants. The unprecedented accumulation of ADP-Glc in the ss3/ss4 plants will have important knock-on effects in the chloroplasts, as the adenosine and phosphate moieties within the ADP-Glc pool will in effect be locked away and unavailable for other metabolic processes. In wild-type plants, ATP and ADP are the dominant adenine nucleotides, with AMP and ADP-Glc forming only a small percentage (about 2% each) of the total adenine nucleotide pool (Fig. 1, E–H; Supplemental Fig. S3). Our measurements of ATP and ADP in wildtype plants indicate a total pool size of around 100 nmol g21 fresh weight, close to the value of 120 nmol g21 fresh weight reported by Strand et al. (2000). This is actually lower than the amount of ADP-Glc alone that we found in the ss3/ss4 and ss4 mutants, and the total adenylate pools in these two mutants are four times higher than in wild-type plants (Supplemental Fig. S3). This suggests that there has been a massive compensatory increase in the total adenine nucleotide pool size in the mutants, but despite this increase, the vast majority (77%–81%) of the enlarged pools are still sequestered in the form of ADP-Glc. As a consequence, there will be very little ADP available in the chloroplast stroma for photophosphorylation, which in turn will have severely negative effects on many metabolic and other processes in the chloroplasts. The most obvious effect of impaired ATP synthesis will be the limitation of photosynthetic CO2 assimilation, as observed in the ss3/ss4 mutant (Fig. 4). Within the Calvin-Benson cycle, ATP is required not only for the regeneration of ribulose-1,5-bisphosphate but also for the synthesis of triose phosphates from 3-phosphoglycerate (Heldt and Heldt, 2006; MacRae and Lunn, 2006). Limiting the synthesis of triose phosphates will, in turn, restrict their export to the cytosol for the synthesis and export of Suc to sink organs such as developing leaves, roots, flowers, and seeds. Low stromal ATP levels will also inhibit many of the biosynthetic pathways that occur in the chloroplasts (e.g. for the production of lipids, amino acids, nucleotides, isoprenoids, phenylpropanoids, and vitamins; Lunn, 2007). Presumably, at some point, ATP will become limiting for the further synthesis of ADP-Glc itself. However, the Km (ATP) of the Arabidopsis AGPase is around 70 mM (Crevillén et al., 2003), so it is capable of drawing down the stromal concentration of ATP to very low levels. ATP is also required for many housekeeping functions in the chloroplasts, including DNA, RNA, and protein synthesis, as well as the import, processing, and assembly of nucleus-encoded proteins. Altogether, the profound disturbance of chloroplast metabolism caused by low ATP availability is bound to have far-reaching effects beyond the chloroplasts, reducing the energy and carbon supplies available for cell maintenance and growth. Low ADP availability in the chloroplasts could also result in damage to existing chloroplast structures due to overenergization of the thylakoid membranes. The reduced rate of photophosphorylation and, therefore, dissipation of the proton-motive force across the thylakoid membranes will lead to hyperpolarization of the membranes, blocking further electron transport between the photosystems (Heldt and Heldt, 2006). This will lower the relative quantum yield of the linear electron transport, leading to a reduction in FPSII, as observed in the ss3/ss4 plants (Table I; Baker, 2008). This situation favors the formation of ROS that can cause oxidative damage to the photosynthetic apparatus (Niyogi, 1999). This is corroborated by the low value of Fv/Fm found in the ss3/ss4 plants, which is indicative of photoinhibition (Maxwell and Johnson, 2000). Further evidence of oxidative stress in the ss3/ss4 plants came from the finding of high levels of MAD in these plants, which is a characteristic product of membrane lipid peroxidation by ROS (Janero, 1990). One of the adaptive responses of plants to photoinhibitory damage is the adjustment of the light-harvesting antenna size in order to balance light absorption and utilization (Niyogi, 1999). This response explains the reduction in the amounts of chlorophylls a and b and carotenoids found in the ss3/ss4 plants (Fig. 2). Finally, the high levels 82 Plant Physiol. Vol. 163, 2013 Downloaded from on June 18, 2017 - Published by www.plantphysiol.org Copyright © 2013 American Society of Plant Biologists. All rights reserved. Growth Defects of ss3/ss4 Linked to High ADP-Glucose of anthocyanins detected in the ss3/ss4 plants provide further support for the conclusion that the ss3/ss4 plants are subject to photoinhibition. Anthocyanin accumulation generally coincides with situations where there is an imbalance between light capture, CO2 assimilation, and carbohydrate utilization (Wand et al., 2002). All of the phenotypic traits described for the ss3/ss4 plants were also detected in the parental ss4 single mutant, such as pale color (Fig. 2; Roldán et al., 2007), lower growth and CO2 assimilation rates (Figs. 3 and 4), and low values of Fv/Fm and FPSII (Table I), although these effects were less severe than in the ss3/ss4 plants. Plants lacking SS4 accumulate around 60% to 80% of the starch found in wild-type plants (Roldán et al., 2007), suggesting that there is greater turnover of the ADP-Glc pool in ss4 than in the ss3/ss4 double mutant. Nevertheless, we observed a similarly large accumulation of ADP-Glc in both mutants, indicating that the limitation of starch synthesis in the single ss4 mutant is sufficient to have a major impact on ADP-Glc levels and to trigger a similar compensatory increase in the total adenylate pool to that seen in the ss3/ss4 mutant. We tested the hypothesis that the chlorotic and growth phenotypes of the ss3/ss4 mutant are attributable to the massive ADP-Glc accumulation by introducing further mutations (aps1 and pgm1) to limit ADP-Glc synthesis. The pgm1 mutation blocks the production of Glc-1-P in the chloroplasts, so there is no substrate available for ADP-Glc synthesis, except for a very small amount imported from the cytosol by a Glc-1-P transporter (Fettke et al., 2011). Introduction of the pgm1 mutation into the ss3/ss4 background lowered the amount of ADP-Glc accumulated in the leaves by 97% and restored the photosynthetic capacity and growth of the plants to nearly wild-type levels (Table II; Figs. 1E, 5, and 7–9). Knocking out the APS1 gene, encoding the small subunit of AGPase, led to an 87% decrease in the accumulation of ADP-Glc in the ss3/ss4 background but was less effective than the loss of PGM activity (Fig. 1E). Nevertheless, the aps1/ss3/ss4 triple mutant grew much better than the ss3/ss4 double mutant and was phenotypically similar to the aps1 parent (Table I; Figs. 5, 7, and 8). Loss of the APS1 subunit does not appear to abolish AGPase activity entirely, because the aps1 mutant was found to contain low but detectable traces of ADP-Glc and a small amount of starch (about 2% of the wildtype level; Fig. 1A). The residual activity can be ascribed to the remaining AGPase large subunits (APL1–APL4), several of which have been demonstrated to have a low level of catalytic activity (Ventriglia et al., 2008). Metabolites were measured in plants harvested after 8 h of illumination. If we assume that all of the ADP-Glc in the aps1/ss3/ss4 mutant (44 nmol g21 fresh weight; Table I) had been synthesized during this period and that there was negligible consumption of ADP-Glc for starch synthesis, we can calculate that as little as 0.09 nmol min21 g21 fresh weight of AGPase activity would be sufficient to account for the observed accumulation of ADP-Glc in the triple mutant. This level of activity would represent less than 0.01% of the maximal catalytic activity of AGPase in wild-type Arabidopsis rosettes (1.0–1.5 mmol min21 g21 fresh weight; Hädrich et al., 2012). The amount of ADP-Glc in the aps1/ss3/ss4 mutant constitutes 34% of the total adenylate pool (Fig. 1E; Supplemental Fig. S3). Thus, by limiting the accumulation of ADP-Glc to a moderate level, a substantial pool of ATP and ADP should remain available for photophosphorylation and other biochemical reactions in the aps1/ss3/ss4 mutant. The photosynthetic capacities and growth rates of the aps1/ss3/ss4 and aps1 mutants were very similar, indicating that even such a partial rebalancing of the adenine nucleotide pool was sufficient to support higher rates of CO2 fixation and growth. Loss of plastidial PGM activity was even more effective at restoring the adenine nucleotide and ADPGlc pools of the pgm/ss3/ss4 mutant to nearly wild-type levels (Fig. 1, E–H). In conclusion, the loss of SS3 and SS4 activity in Arabidopsis leaves almost totally abolishes starch synthesis due to the plant’s inability to initiate starch granules in most of the cells. The block in starch synthesis was found to result in an unprecedented accumulation of ADP-Glc, thereby sequestering much of the plastidial adenine nucleotide in a metabolically inaccessible form. This will severely limit the synthesis and availability of ATP for photosynthetic CO2 fixation and many other processes and will also trigger ROS production, leading to photooxidative damage of the photosynthetic apparatus within the chloroplasts. Restricting the synthesis of ADP-Glc in the chloroplasts by introducing the pgm1 or aps1 mutation into ss3/ss4 greatly reduced the accumulation of ADP-Glc and restored both the photosynthetic capacity and growth of the plants. This confirmed that the massive accumulation of ADPGlc in the ss3/ss4 mutant underlies the chlorotic and stunted growth phenotype of this mutant and explains why it differs so markedly from other starch-deficient mutants. MATERIALS AND METHODS Plant Materials and Growth Conditions All Arabidopsis (Arabidopsis thaliana) mutants were in the Columbia-0 (Col-0) background. The ss3, ss4, and ss3/ss4 mutants were as described by Szydlowski et al. (2009), and the pgm1 mutant was from Caspar et al. (1985). The aps1 mutant (SALK_ 040155) contains a transfer DNA insertion in the APS1 gene (At5g48300; Alonso et al., 2003), and homozygous knockout plants were selected using PCR-based genotyping. Lines carrying triple mutations were obtained by crossing and selecting homozygous triple mutant plants from the segregating F2 populations using PCR-based genotyping. All primers used are described in Supplemental Table S1. Unless otherwise specified, plants were grown in growth cabinets at 23°C (day)/20°C (night), 70% humidity, and an irradiance of 120 mE m22 s21 supplied by white fluorescent lamps. Seeds were sown in soil and irrigated with 0.53 Murashige and Skoog medium (Murashige and Skoog, 1962). Photosynthesis Measurements Photosynthetic gas exchange was measured using a portable infrared gas analyzer (model LI-6400; LI-COR Biosciences), which allows environmental conditions inside a standard leaf clamp of the chamber to be precisely controlled. Plant Physiol. Vol. 163, 2013 83 Downloaded from on June 18, 2017 - Published by www.plantphysiol.org Copyright © 2013 American Society of Plant Biologists. All rights reserved. Ragel et al. Air temperature in the chamber was set at 25°C, and the relative humidity was maintained at 50%. Light-response curves were determined on one of the upper leaves of the plants, progressively increasing the irradiance from 0 to 50 and then to 100, 250, 500, 750, 1,000, 1,500, and 2,000 mmol quanta m22 s21 in stepwise changes every 3 min. The CO2 assimilation rate was determined on 3-week-old plants grown in a controlled environment under LD conditions (16 h of light/8 h of dark) with an irradiance of 100 mmol quanta m22 s21 at a constant 22°C temperature. Photosyn Assistant software, developed by Dundee Scientific (R. Parsons and S.A. Ogston), has been used to determine parameters such as CO2 assimilation rates to help in the comparison between the mutants. Gas exchange of whole Arabidopsis rosettes was measured with a custombuilt multichamber system connected in parallel to an infrared gas analyzer (Li-7000; LI-COR). Individual plants were introduced into each of eight chambers, and after an adaptation period of 2 d, gas exchange was measured for a complete 24-h light/dark period. During the measurement, air with 380 mL L21 CO2 and a relative humidity of 60% was directed through the system. Each chamber was measured consecutively for 6 min, and the average value was taken for the light period. At the end of the experiment, a photograph was taken to calculate the projected leaf area for each plant. Based on the area, photosynthetic and respiration rates were calculated with the ΔCO2 and ΔH2O values gained from the gas-exchange system. Chlorophyll fluorescence emission parameters were measured at 22°C with a PAM 2000 fluorometer (Walz). The Fv/Fm was calculated from the measured parameters using the following equation: Fv/Fm = (Fm 2 Fo)/Fm, where Fo is the initial minimal fluorescence emitted from leaves dark adapted for 1 h and Fm is the maximal fluorescence elicited by saturating actinic light. The FPSII was calculated from the measured parameters using the following equation: FPSII = Fm9 2 F9/Fm9, where Fm9 is the maximal fluorescence from lightadapted leaves and F9 is the fluorescence emission from light-adapted leaves. Plant Growth Measurement Plant growth was determined by weighing the aerial part of the plants. In the case of pgm1 and pgm1/ss3/ss4 mutant plants, the plant growth was measured based on whole rosette area. Digital images were taken every 2 or 3 d at the same time. The digital images were converted with Photoshop (Adobe Photoshop CS4 Extended; Adobe Systems) to black-and-white images, with the rosette being black and the background being white. ImageJ 1.42q (National Institutes of Health) was used to calculate the leaf area, based on the scale, from the black-and-white images. Analytical Methods Seven discs of rosette leaves (5–10 mg) from independent plants were used for the determination of chlorophylls a and b and carotenoids. Pigments were extracted with methanol and quantified according to the methods described by Porra et al. (1989) for chlorophylls and by Lichtenthaler and Buschmann (2001) for carotenoids. The extraction and quantitation of anthocyanins were performed as described (Rabino and Mancinelli, 1986) using seven rosette leaves from independent plants. The concentration of MAD in plants was determined using the thiobarbituric acid method described by Buege and Aust (1978). Soluble sugars and starch were measured enzymatically in ethanolic extracts and the ethanol-insoluble residue as described by Hendriks et al. (2003). ADP-Glc, phosphorylated intermediates, and organic acids were measured in chloroform-methanol extracts using high-pressure anion-exchange chromatography coupled to MS/MS as described (Lunn et al., 2006). ATP, ADP, and AMP were measured enzymatically in freshly prepared TCA extracts as described (Weiner et al., 1987; Trethewey et al., 1998). Supplemental Data The following materials are available in the online version of this article. Supplemental Figure S1. Determination of levels of phosphorylated intermediates and organic acids of wild-type, ss3, ss4, ss3/ss4, aps1, pgm1, aps1/ss3/ss4, and pgm1/ss3/ss4 Arabidopsis plants grown in 16-h-light/ 8-h-dark conditions (20°C/18°C, irradiance of 150 mE m22 s21) and harvested at 8 h into the light period. Supplemental Figure S2. Growth rates of Col-0, aps1/ss3/ss4, pgm1/ss3/ss4, and their parental lines. Supplemental Figure S3. Total adenine nucleotide pool size (ATP + ADP + AMP + ADP-Glc) was measured in wild-type, ss3, ss4, ss3/ss4, aps1, aps1/ ss3/ss4, pgm, and pgm/ss3/ss4 Arabidopsis plants grown in 16-h-light/ 8-h-dark conditions (20°C/18°C, irradiance at 150 mE m22 s21) and harvested at 8 h into the light period. Supplemental Table S1. Primers used for the identification of the triple mutant plants. Received June 17, 2013; accepted July 18, 2013; published July 21, 2013. LITERATURE CITED Alonso JM, Stepanova AN, Leisse TJ, Kim CJ, Chen H, Shinn P, Stevenson DK, Zimmerman J, Barajas P, Cheuk R, et al (2003) Genome-wide insertional mutagenesis of Arabidopsis thaliana. Science 301: 653–657 Bahaji A, Li J, Ovecka M, Ezquer I, Muñoz FJ, Baroja-Fernández E, Romero JM, Almagro G, Montero M, Hidalgo M, et al (2011) Arabidopsis thaliana mutants lacking ADP-glucose pyrophosphorylase accumulate starch and wild-type ADP-glucose content: further evidence for the occurrence of important sources, other than ADP-glucose pyrophosphorylase, of ADP-glucose linked to leaf starch biosynthesis. Plant Cell Physiol 52: 1162–1176 Baker NR (2008) Chlorophyll fluorescence: a probe of photosynthesis in vivo. Annu Rev Plant Biol 59: 89–113 Ball S, Colleoni C, Cenci U, Raj JN, Tirtiaux C (2011) The evolution of glycogen and starch metabolism in eukaryotes gives molecular clues to understand the establishment of plastid endosymbiosis. J Exp Bot 62: 1775–1801 Ballicora MA, Iglesias AA, Preiss J (2003) ADP-glucose pyrophosphorylase, a regulatory enzyme for bacterial glycogen synthesis. Microbiol Mol Biol Rev 67: 213–225 Buege JA, Aust SD (1978) Microsomal lipid peroxidation. Methods Enzymol 52: 302–310 Caspar T, Huber SC, Somerville C (1985) Alterations in growth, photosynthesis, and respiration in a starchless mutant of Arabidopsis thaliana (L.) deficient in chloroplast phosphoglucomutase activity. Plant Physiol 79: 11–17 Crevillén P, Ballicora MA, Mérida A, Preiss J, Romero JM (2003) The different large subunit isoforms of Arabidopsis thaliana ADP-glucose pyrophosphorylase confer distinct kinetic and regulatory properties to the heterotetrameric enzyme. J Biol Chem 278: 28508–28515 Delvallé D, Dumez S, Wattebled F, Roldán I, Planchot V, Berbezy P, Colonna P, Vyas D, Chatterjee M, Ball S, et al (2005) Soluble starch synthase I: a major determinant for the synthesis of amylopectin in Arabidopsis thaliana leaves. Plant J 43: 398–412 D’Hulst C, Mérida Á (2012) Once upon a prime: inception of the understanding of starch initiation in plants. In IJ Tetlow, ed, The Synthesis and Breakdown of Starch (Essential Reviews in Experimental Biology), Society of Experimental Biology, London, Vol 5. pp 55–76 Fettke J, Malinova I, Albrecht T, Hejazi M, Steup M (2011) Glucose-1phosphate transport into protoplasts and chloroplasts from leaves of Arabidopsis. Plant Physiol 155: 1723–1734 Gibon Y, Bläsing OE, Palacios-Rojas N, Pankovic D, Hendriks JHM, Fisahn J, Höhne M, Günther M, Stitt M (2004) Adjustment of diurnal starch turnover to short days: depletion of sugar during the night leads to a temporary inhibition of carbohydrate utilization, accumulation of sugars and post-translational activation of ADP-glucose pyrophosphorylase in the following light period. Plant J 39: 847–862 Hädrich N, Hendriks JHM, Kötting O, Arrivault S, Feil R, Zeeman SC, Gibon Y, Schulze WX, Stitt M, Lunn JE (2012) Mutagenesis of cysteine 81 prevents dimerization of the APS1 subunit of ADP-glucose pyrophosphorylase and alters diurnal starch turnover in Arabidopsis thaliana leaves. Plant J 70: 231–242 Heldt HW, Heldt F (2006) Plant Biochemistry, Ed 3. Elsevier Academic Press, Oxford Hendriks JH, Kolbe A, Gibon Y, Stitt M, Geigenberger P (2003) ADPglucose pyrophosphorylase is activated by posttranslational redoxmodification in response to light and to sugars in leaves of Arabidopsis and other plant species. Plant Physiol 133: 838–849 Janero DR (1990) Malondialdehyde and thiobarbituric acid-reactivity as diagnostic indices of lipid peroxidation and peroxidative tissue injury. Free Radic Biol Med 9: 515–540 84 Plant Physiol. Vol. 163, 2013 Downloaded from on June 18, 2017 - Published by www.plantphysiol.org Copyright © 2013 American Society of Plant Biologists. All rights reserved. Growth Defects of ss3/ss4 Linked to High ADP-Glucose Lichtenthaler HK, Buschmann C (2001) Chlorophylls and carotenoids: measurement and characterization by UV-VIS spectroscopy. In RE Wrolstad, TE Acree, EA Decker, MH Penner, DS Reid, SJ Schwartz, CF Shoemaker, D Smith, P Sporns, eds, Current Protocols in Food Analytical Chemistry. John Wiley & Sons, Hoboken, NJ, unit F4.3, supplement 1 Lin TP, Caspar T, Somerville C, Preiss J (1988a) Isolation and characterization of a starchless mutant of Arabidopsis thaliana (L.) Heynh lacking ADP-glucose pyrophosphorylase activity. Plant Physiol 86: 1131–1135 Lin TP, Caspar T, Somerville CR, Preiss J (1988b) A starch deficient mutant of Arabidopsis thaliana with low ADP-glucose pyrophosphorylase activity lacks one of the two subunits of the enzyme. Plant Physiol 88: 1175–1181 Lunn JE (2007) Compartmentation in plant metabolism. J Exp Bot 58: 35–47 Lunn JE, Feil R, Hendriks JHM, Gibon Y, Morcuende R, Osuna D, Scheible W-R, Carillo P, Hajirezaei M-R, Stitt M (2006) Sugar-induced increases in trehalose 6-phosphate are correlated with redox activation of ADPglucose pyrophosphorylase and higher rates of starch synthesis in Arabidopsis thaliana. Biochem J 397: 139–148 MacRae EA, Lunn JE (2006) Control of sucrose biosynthesis. In WC Plaxton, MT McManus, eds, Advances in Plant Research, Vol 22: Control of Primary Metabolism. Blackwell, Oxford, pp 234–257 Maxwell K, Johnson GN (2000) Chlorophyll fluorescence: a practical guide. J Exp Bot 51: 659–668 Murashige T, Skoog F (1962) A revised medium for rapid growth and bioassays with tobacco tissue cultures. Plant Physiol 15: 473–497 Niyogi KK (1999) Photoprotection revisited: genetic and molecular approaches. Annu Rev Plant Physiol Plant Mol Biol 50: 333–359 Porra R, Thompson W, Kriedemann P (1989) Determination of accurate extinction coefficients and simultaneous equations for assaying chlorophylls a and b extracted with four different solvents: verification of the concentration of chlorophyll standards by atomic absorption spectroscopy. Biochim Biophys Acta 975: 384–394 Rabino I, Mancinelli AL (1986) Light, temperature, and anthocyanin production. Plant Physiol 81: 922–924 Roldán I, Wattebled F, Mercedes Lucas M, Delvallé D, Planchot V, Jiménez S, Pérez R, Ball S, D’Hulst C, Mérida A (2007) The phenotype of soluble starch synthase IV defective mutants of Arabidopsis thaliana suggests a novel function of elongation enzymes in the control of starch granule formation. Plant J 49: 492–504 Schulze W, Stitt M, Schulze ED, Neuhaus HE, Fichtner K (1991) A quantification of the significance of assimilatory starch for growth of Arabidopsis thaliana L. Heynh. Plant Physiol 95: 890–895 Smith AM, Stitt M (2007) Coordination of carbon supply and plant growth. Plant Cell Environ 30: 1126–1149 Stitt M, Zeeman SC (2012) Starch turnover: pathways, regulation and role in growth. Curr Opin Plant Biol 15: 282–292 Strand A, Zrenner R, Trevanion S, Stitt M, Gustafsson P, Gardeström P (2000) Decreased expression of two key enzymes in the sucrose biosynthesis pathway, cytosolic fructose-1,6-bisphosphatase and sucrose phosphate synthase, has remarkably different consequences for photosynthetic carbon metabolism in transgenic Arabidopsis thaliana. Plant J 23: 759–770 Streb S, Egli B, Eicke S, Zeeman SC (2009) The debate on the pathway of starch synthesis: a closer look at low-starch mutants lacking plastidial phosphoglucomutase supports the chloroplast-localized pathway. Plant Physiol 151: 1769–1772 Sun J, Okita TW, Edwards GE (1999) Modification of carbon partitioning, photosynthetic capacity, and O2 sensitivity in Arabidopsis plants with low ADP-glucose pyrophosphorylase activity. Plant Physiol 119: 267–276 Szydlowski N, Ragel P, Raynaud S, Lucas MM, Roldán I, Montero M, Muñoz FJ, Ovecka M, Bahaji A, Planchot V, et al (2009) Starch granule initiation in Arabidopsis requires the presence of either class IV or class III starch synthases. Plant Cell 21: 2443–2457 Trethewey RN, Geigenberger P, Riedel K, Hajirezaei MR, Sonnewald U, Stitt M, Riesmeier JW, Willmitzer L (1998) Combined expression of glucokinase and invertase in potato tubers leads to a dramatic reduction in starch accumulation and a stimulation of glycolysis. Plant J 15: 109–118 Ventriglia T, Kuhn ML, Ruiz MT, Ribeiro-Pedro M, Valverde F, Ballicora MA, Preiss J, Romero JM (2008) Two Arabidopsis ADP-glucose pyrophosphorylase large subunits (APL1 and APL2) are catalytic. Plant Physiol 148: 65–76 Wand S, Holcroft D, Jacobs G (2002) Anthocyanins in vegetative tissues: a proposed unified function in photoprotection. New Phytol 155: 349–361 Weiner H, Stitt M, Heldt HW (1987) Subcellular compartmentation of pyrophosphate and alkaline pyrophosphatase in leaves. Biochim Biophys Acta 893: 13–21 Zhang X, Myers AM, James MG (2005) Mutations affecting starch synthase III in Arabidopsis alter leaf starch structure and increase the rate of starch synthesis. Plant Physiol 138: 663–674 Plant Physiol. Vol. 163, 2013 85 Downloaded from on June 18, 2017 - Published by www.plantphysiol.org Copyright © 2013 American Society of Plant Biologists. All rights reserved.