* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download X-Chromosome inactivation ratios affect wild-type
Survey
Document related concepts
Polycomb Group Proteins and Cancer wikipedia , lookup
Gene therapy of the human retina wikipedia , lookup
Nutriepigenomics wikipedia , lookup
Epigenetics of neurodegenerative diseases wikipedia , lookup
Site-specific recombinase technology wikipedia , lookup
Transcript
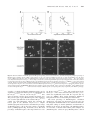
Human Molecular Genetics, 2004, Vol. 13, No. 12 DOI: 10.1093/hmg/ddh142 Advance Access published on April 28, 2004 1275–1286 X-Chromosome inactivation ratios affect wild-type MeCP2 expression within mosaic Rett syndrome and Mecp2 2/1 mouse brain Daniel Braunschweig, Thomas Simcox, Rodney C. Samaco and Janine M. LaSalle* Medical Microbiology and Immunology, Rowe Program in Human Genetics, School of Medicine, 1 Shields Avenue, University of California, Davis, CA 95616, USA Received March 14, 2004; Revised April 5, 2004; Accepted April 15, 2004 Rett syndrome (RTT) is an X-linked neurodevelopmental disorder caused by mutations in MECP2, encoding methyl-CpG-binding protein 2 (MeCP2). The onset of symptoms in RTT is delayed until 6 – 18 months and 4 –6 months in the Mecp2 2/1 mouse model, corresponding to a dynamic and gradual accumulation of MeCP2 expression in individual neurons of the postnatal brain. Because of X chromosome inactivation (XCI), cells within RTT females are mosaic for expression of the heterozygous MECP2 mutation. Using the targeted Mecp2 mouse model, we investigated the effect of Mecp2 mutation on XCI and developmental MeCP2 expression in wild-type (wt)-expressing neurons by quantitative laser scanning cytometry. Mecp2 2/1 female mice exhibited uniform regional distribution of Mecp2 mutant-expressing cells in brain, but unbalanced XCI in the population, favoring expression of the Mecp2 wt allele. Interestingly, MeCP2 expression in Mecp2 wt-expressing cells from Mecp2 2/1 mice was significantly lower than those from Mecp2 1/1 agematched controls. The negative effect of Mecp2 mutation on wt Mecp2 expression correlated with the percentage of Mecp2 mutant-expressing cells in the cortex. Similar results were observed in two RTT females with identical MECP2 mutations but different XCI ratios. These results demonstrate that Mecp2-mutant neurons affect the development of surrounding neurons in a non-cell-autonomous manner and suggest that environmental influences affect the level of MeCP2 expression in wt neurons. These results help in explaining the role of XCI in the pathogenesis of RTT and have important implications in designing therapies for female RTT patients. INTRODUCTION Rett syndrome (RTT) is an X-linked neurodevelopmental disorder and a common genetic cause of severe mental retardation in females (1,2). Clinical features are not manifested until around 6 –18 months of age and include the loss of vocalization skills and purposeful hand movements, deceleration of head growth, ataxia, autistic features, seizures and breathing abnormalities (3). RTT is caused by mutations in MECP2, encoding methyl-CpG-binding protein 2 (MeCP2) (4). Although MECP2 is ubiquitously expressed at the transcriptional level (5 – 7), tissue-specific and developmental regulation of MECP2 transcription and alternate polyadenylation result in elevated MeCP2 expression limited to a subpopulation of neurons in the postnatal central nervous system (CNS) (8 – 10). The MeCP2 binds to methylated DNA (11), localizes to nuclear heterochromatin (12) and is predicted to be a transcriptional repressor of methylated genes (13). Several mouse models for RTT have been produced by creating null or truncating mutations of Mecp2. Two groups produced Mecp2-null mice by cre –lox recombinase technology that were targeted to either the whole mouse or restricted to postmitotic neurons in the CNS (14,15). The onset of neurologic symptoms was delayed until 3– 6 weeks in the Mecp2 2/y male mice and 4 –6 months in the Mecp2 2/þ female mice, with lethality resulting by 10 weeks of age in the males. The conditional neuronal Mecp2-null mice had the same phenotype, with a slightly delayed age of onset. A truncated Mecp2 308/y mutant mouse also displayed many features of RTT, including motor impairments, seizures, autistic behaviors and hypoactivity (16). The phenotype of the Mecp2 308/y mice was less severe than that of the Mecp2-null mice and *To whom correspondence should be addressed at: Medical Microbiology and Immunology, Rowe Program in Human Genetics, School of Medicine, 1 Shields Avenue, University of California, Davis, CA 95616, USA. Tel: þ1 5307547598; Fax: þ1 5307528692; Email: [email protected] Human Molecular Genetics, Vol. 13, No. 12 # Oxford University Press 2004; all rights reserved 1276 Human Molecular Genetics, 2004, Vol. 13, No. 12 was non-lethal. Furthermore, the Mecp2 3082/þ females displayed variability and delayed onset of symptoms compared with the Mecp2-null models or RTT females. Overall these mouse models appear to recapitulate the phenotype of RTT in the mouse, although a major question that remains is why the onset of symptoms in female Mecp2 2/þ mice is delayed until adulthood in the mouse but occurs during infancy in humans. One hypothesis is that MeCP2 is required for neuronal maintenance rather than maturation and therefore the onset of symptoms may follow a chronological rather than developmental time line (14,15). Another possibility is that MeCP2 expression is developmentally regulated and not required until postnatal CNS development during synaptogenesis (8 – 10,17). In humans, MeCP2 protein expression shows complex developmental and tissue-specific heterogeneity (8 – 10), with a population of high-expressing (MeCP2hi) neurons emerging in juveniles and continuing through adulthood in the cerebral cortex (10). Heterogeneity in the level of MeCP2 within the CNS has also been observed in the macaque (18), rat (19,20) and mouse (9,17). In mouse, developmental expression has been investigated previously by immunoblot and immunohistochemistry, demonstrating a relatively stable expression during postnatal brain development (9). Analysis of MeCP2 by immunoblot in isolated olfactory bulb (17) or cerebellum (20) demonstrated increased expression in adulthood, however, suggesting that changes in MeCP2 expression may be evident within specific brain regions. Although these previous studies have suggested a role for MeCP2 in the maturational differentiation of CNS neurons based on the timing of its expression, a quantitative analysis of MeCP2 expression in different brain regions during development has not been performed previously. In addition to developmental regulation of MeCP2, X chromosome inactivation (XCI) plays an important role in the onset and severity of RTT symptoms (2). As MECP2/ Mecp2 is located on the X chromosome, female RTT patients and Mecp2 2/þ mice are mosaic for expression of MECP2/ Mecp2 mutations. Although most RTT patients show random XCI ratios in brain and blood (21 –23), some asymptomatic carriers of MECP2 mutations show extremely skewed XCI (24). A recent report on XCI in the Mecp2 308/þ mouse model demonstrated unbalanced XCI patterns favoring expression of the wild-type (wt) allele that correlated with phenotypic outcome (25). As MECP2 mutant cells are randomly distributed in the RTT brain (8), we hypothesized that non-cell-autonomous mechanisms may influence the level of MeCP2 expression in wt-expressing neurons. In this study, we perform quantitative localization of MeCP2 expression in sagittal mouse brain sections by laser scanning cytometry (LSC). The LSC is a new technology that not only quantifies immunofluorescence of contoured cells within tissue sections, but also localizes each cell back to the original tissue position (8). In this report, we use LSC to demonstrate dynamic regional and developmental expression and localization patterns of MeCP2 in brain that were not fully evident by previous methodologies. In addition, the combined use of LSC with tissue microarrays allowed us to quantitate MeCP2 expression in subpopulations of cells from multiple Mecp2 2/þ and control tissues on the same slide. This quantitative approach allowed us to observe an effect of Mecp2 mutation on expression of the wt allele in Mecp2 2/þ mice that was not previously apparent. These results suggest a model for the pathogenesis of RTT in which the normal dynamic developmental expression and function of MeCP2 is altered by MECP2 mutation by both cell autonomous and non-autonomous mechanisms. RESULTS Quantitative localization of MeCP2 in the developing mouse brain reveals dynamic and regional patterns of expression Because of previous evidence of complex regulation of MeCP2 expression in different cellular subpopulations and brain regions during mammalian brain development (8,9,17), we decided to use LSC to quantitatively localize MeCP2 expression in whole mouse brain sections at 21 different time points during embryonic and postnatal development. The LSC analysis measures fluorescent intensity of individual cells within tissue sections contoured on the basis of a nuclear counterstain, as described previously (8). Figure 1 shows representative analyses of eight different time points in which consecutive sagittal sections taken 1 mm from the midline were stained with hemotoxylin and eosin (H&E) to identify brain regions within the x, y position scattergrams (XY Pos) generated by LSC analysis that represent the slide position of each contoured cell. Each slide was scanned using identical non-saturating settings for the long red (Cy5 fluorescence) photomultiplier tube (PMT) so that quantitative comparisons could be made between different time points. Cell populations were gated and colored on the basis of MeCP2 ‘max pixel’ histograms of whole brain. Max pixel is the maximum fluorescent intensity of each contoured nucleus, as described previously (8). Region 1 (blue) reflects the low, but positive MeCP2 level observed in the late embryonic time points. Regions 2 and 3 are the two dominant populations found in the adult, reflecting the MeCP2lo (green) and MeCP2hi (red) populations, respectively, as described previously (8). The colored populations are represented on the LSC scattergram of x, y position and show regional differences and changes in regional distribution throughout development. In order to quantitate regional differences in MeCP2 fluorescence, six different identifiable brain regions (olfactory bulb, cerebral cortex, hippocampus, cerebellum, medulla and thalamus) were separately gated on the x, y scattergram (XY Gated). Regional analysis demonstrated that high MeCP2 fluorescence in the neonatal mouse brain was limited to the medulla and thalamus. By 1– 2 weeks of age, however, there was a dramatic decrease in MeCP2 fluorescence for both medulla and thalamus that was primarily responsible for the overall decrease in the whole brain from 1 to 2 weeks of age. From around 3 to 40 weeks of age, the MeCP2hi population (red) showed a gradual reemergence in all regions of the brain. A graphical representation of the developmental changes in mean MeCP2 fluorescence of all contoured nuclei within the whole brain for all 21 time points is shown in Figure 2A. Each data point represents the mean+SEM from four replicates of at least two mice for each time point. A Human Molecular Genetics, 2004, Vol. 13, No. 12 1277 Figure 1. Dynamic regional expression of MeCP2 in the developing mouse brain revealed by laser scanning cytometry (LSC). Wild type (wt) C56BL/6 mouse brains were fixed at 21 different embryonic and postnatal ages, sagittally sectioned, and analyzed for quantitative localization of MeCP2 immunofluorescence (Cy5 detection) of individual cells by LSC. Eight representative time points are shown to demonstrate full LSC analysis, while mean MeCP2 levels for whole brain analyses of all samples and time points are graphed in Figure 2A. For each time point, the hemotoxylin and eosin (H&E) stained parallel slide was used to determine the tissue localization of cells within the LSC scattergram of x,y position (XY Pos). For embryonic and early postnatal time points, the whole head was sectioned and the brain region gated on the x,y position scattergram; black regions represent non-brain cells that were excluded from analysis. Histograms representing MeCP2 fluorescence (x-axis) and cell count (y-axis) for the whole mouse brain section (Whole Brain) are shown for LSC analyses with equivalent long red PMT detection for all time points to quantitate developmental differences in MeCP2 expression. Histograms are gated and colored into five regions based on MeCP2 fluorescence: region 1 (blue) represents the late embryonic expression of MeCP2, set at the right half max of the E16 time point: region 2 (green) represents the previously described MeCP2lo population, set at the right half max of the adult P40 week time point; region 3 (red) represents the previously described MeCP2hi population, and includes the remainder of the cells. In order to examine regional differences in these populations, different anatomical structures identified from H&E staining were gated on the x,y position scattergram (XY Gated). Histograms of MeCP2 fluorescence (x-axis) and cell count (y-axis) for each separately gated region are shown for each time point. The total of number of cells contoured are shown for each histogram. Dynamic changes were observed in MeCP2 expression-based populations in different brain regions throughout development. A large population of MeCP2hi cells was observed in the perinatal medulla and thalamus that decreased by 1 week of age and gradually re-emerged from 4 to 40 weeks of age in all brain regions. 1278 Human Molecular Genetics, 2004, Vol. 13, No. 12 Human Molecular Genetics, 2004, Vol. 13, No. 12 3-fold increase in MeCP2 max pixel mean was observed from the earliest (E10 or 29d) to the latest (E19 or 0d) embryonic time point. This was followed by a rapid equivalent decrease in early postnatal life, then a rapid 3-fold increase from 5d to 21 days. From around 3 to 40 weeks (21 – 280 days) of postnatal life, there was a gradual 2-fold increase in MeCP2 max pixel mean, reflecting the accumulation of the MeCP2hi population in all regions of the brain, as demonstrated in Figure 1. As the max pixel measurement for quantitating MeCP2 immunofluorescence used in Figures 1 and 2A could reflect changes in localization of MeCP2 within the nucleus as well as the level of MeCP2 expression, we sought to determine MeCP2 expression and nuclear localization by additional LSC analyses and immunoblot. The LSC analysis of long red integral is the sum of all the pixel intensities inside the nuclear contour (similar to the ‘area under the curve’ measurement commonly used in image quantitation) and reflects the total amount of MeCP2. To normalize for cell size, the MeCP2 ‘integral’ was divided by the nuclear area for each contoured cell and the results from the whole brain analyses are plotted in Figure 2B (black circles and solid line). A .4-fold spike was still observed in the neonatal brain for the MeCP2 integral over nuclear area measurement, but unlike the MeCP2 max pixel measurement in Figure 2A, no increase was observed from 1 to 3 weeks of age. These results suggested that the second increase in MeCP2 max pixel mean observed from 5 to 21 days in Figure 2A was due to MeCP2 localization changes and not due to an increase in the overall expression. Interestingly, there was a corresponding increase in the nuclear area (open squares and dotted line) during the first 2 weeks of postnatal age, suggesting that global nuclear organization changes occurring during this time frame may reflect the difference in the two analyses. Immunoblot analysis of MeCP2 expression using protein extracted from whole mouse brains at 17 different time points was performed as an independent method (Fig. 2C). Following detection with anti-MeCP2, blots were re-probed with anti-GAPDH for semi-quantitative analysis of MeCP2 expression. The quantitative results from four different immunoblots are shown in Figure 2D, demonstrating a similarly rapid 5-fold increase in MeCP2 expression in the perinatal 1279 brain as was observed for the analysis of MeCP2 integral normalized to nuclear area in Figure 2B (closed circles and solid line). In contrast, the gradual 2-fold increase in MeCP2 expression observed by LSC analysis from 3 to 40 weeks (Fig. 2A and B) was not observed by immunoblot analysis (Fig. 2D). The LSC’s ability to quantitate expression levels of individual cells unlike the whole protein analysis by immunoblot may explain the discrepancy. These combined results demonstrate that the dynamic changes observed in MeCP2 fluorescence observed by LSC reflect changes in both the level of protein expression and the changes in nuclear localization of heterochromatin. Mecp2 2/1 mice show uniform distribution of MeCP2negative cells in brain Because of the important influence XCI ratios have on the development of RTT in humans (22), we were interested in quantitating and localizing the Mecp2 mutant-expressing cells within females heterozygous for the Mecp2-null mutation. Two different Mecp2-null strains were used for these studies. The Mecp2 tm1.1Bird strain has excised Mecp2 exons 3 and 4 (15), while the Mecp2 tm1.1Jaestrain has only exon 3 deleted (14). Male Mecp2 2/y mice were used as a negative control for immunofluorescence as they have been determined previously to have an absence of MeCP2 expression (14,15). Consistent with this expectation, Mecp2 tm1.1Bird/y mice had a level of MeCP2 immunofluorescence equivalent to the immunoglobulin G (IgG) control (Fig. 3A) and were used to set the region for determining the MeCP2-negative population. For whole brain regional analysis of MeCP2-negative cells in heterozygous females, sagittal brain sections from two Mecp2 tm1.1Bird/þ mice were analyzed for regional MeCP2 expression as in Figure 1. A graph of the combined results of the MeCP2-negative cells for different brain regions is shown in Figure 3B. Although there existed some variation in the percentage of MeCP2negative cells in differently gated brain regions for each individual, no region was significantly different than the whole brain by t-test. These results demonstrate uniform regional distribution of MeCP2-negative cells in the mouse brain. Figure 2. Quantitation of MeCP2 expression versus nuclear localization changes in whole mouse brain by LSC and immunoblot analyses. (A) Quantitation of developmental changes in MeCP2 fluorescent intensity by LSC max pixel analysis of whole sagittal brain sections for 20 different time points. Long red max pixel represents the highest intensity pixel within the contoured nuclei and is influenced by localization of MeCP2. The mean long red max pixel from the histograms in Figure 1 (Whole Brain) are plotted on the y-axis, with chronological age in days on the x-axis. The results are graphed as the mean + SEM of four replicate experiments from at least two mice per time point. A sharp rise in the MeCP2 max pixel mean was observed in the late embryonic to early postnatal time points (21 to 1d), followed by a decline in the first week of life, a second rise from 1 week to 3 weeks, then a gradual rise from 4 to 40 weeks. (B) The LSC quantitation of total levels of MeCP2 per nucleus compared with nuclear area. The LSC analysis of MeCP2 integral values divided by nuclear area for each contoured nucleus is plotted as filled circles, representing the mean + SEM and solid trendline. Long red integral is the addition of all pixel intensities within the nuclear contour, normalized for cell size by dividing by the nuclear area. The first peak of MeCP2 expression in perinatal development is the most pronounced change, while the second increase in MeCP2 max pixel values observed from 1 to 3 weeks in A was not observed. Nuclear area measurements are also plotted (open squares and dotted trendline) and demonstrate a major increase from P1 to P10, with relatively stable values beyond this time frame. The coincidence of nuclear area increase and MeCP2 max pixel values suggests that changes in MeCP2 localization to large nuclear heterochromatin sites occurs during this time frame. However, a gradual 2-fold increase in MeCP2 integral normalized to nuclear area was also observed from P10 to P280, consistent with the emergence of the MeCP2hi population, suggesting that changes in expression and localization of MeCP2 occur during mouse adulthood. (C) Immunoblot analysis of whole mouse brain MeCP2 expression levels was performed for 17 different time points. MeCP2 was observed as a single 76 kDa band in all lanes but relative expression varied with development. The blot was re-probed with an antibody to glyceraldehyde 3-phosphate dehydrogenase (GAPDH) as a control, which ran as a single 37 kDa band. (D) Band intensities from three replicate immunoblots were quantitated and plotted as MeCP2/GAPDH (y-axis) versus chronologic age (xaxis). The solid trendline appears similar to the solid line in B representing the LSC total nuclear MeCP2 measurements except the gradual 2-fold increase was not observed from P10 to P280. Thus, the LSC may be more sensitive to detecting MeCP2 expression differences because of the sampling of individual cells. Trendlines were plotted based on a moving average of two points on each timecourse. 1280 Human Molecular Genetics, 2004, Vol. 13, No. 12 Figure 3. Precise determination of MeCP2-negative populations in two Mecp2-null mouse models and regional distribution in whole brain. (A) The LSC analysis of cerebral cortical samples containing the different genotypes of two different RTT mouse models was performed at both saturating and non-saturating settings. Saturating conditions were used to compare Mecp2 2/y samples to the IgG negative control (black overlay) so that the region for determining the MeCP2-negative population (region 1, gray) could be set (top). The middle panel shows representative histograms from female Mecp2 2/þ mice in which the percentage of MeCP2-negative population was determined using region 1 and saturating conditions so that the two expression peaks were noticeably separate. The bottom panel shows the same female samples under non-saturating settings. Region 1 was set to include the percentage of MeCP2-negative population previously determined for each tissue. Cells in region 2 (MeCP2-positive) were separately gated to obtain the values for MeCP2 and histone H1 expression in Figure 5. (B) Brain regional localization of MeCP2-negative populations was determined from whole brain sagittal sections of two Mecp2 tm1.1Bird/þ mice with 30% MeCP2-negative cells. Regional gating was performed as in Figure 1, and the results shown are the mean + SEM of the two samples for each region. No significant differences were observed in the distribution of MeCP2-negative cells in any region compared with the whole brain. The percentage of MeCP2-negative calculated from each Mecp2 2/1 brain sample inversely correlates with MeCP2 expression in wt-expressing cells If Mecp2/MECP2 mutations acted by a completely cellautonomous mechanism in mosaic females, only cells expressing the mutant allele would exhibit a phenotype. In light of the complex regulation of MeCP2 expression in postnatal development, we hypothesized that MeCP2 expression levels and/or localization may be affected in the MeCP2-positive expressing cells of females heterozygous for MECP2/Mecp2 mutation. To test the hypothesis in mouse, a tissue microarray was prepared containing triplicate cores of cerebral cortex from five Mecp2 tm1.1Bird/þ, five Mecp2 tm1.1Jae/þ, and three Mecp2 þ/þ controls (P20 –27w); one Mecp2 tm1.1Bird/y, and one Mecp2 þ/y littermate (P10w); one Mecp2 tm1.1Jae/y and one Mecp2 þ/y littermate control (P10w). Serial sections derived from tissue microarrays were analyzed for C-terminal anti-MeCP2 (four replicates), rabbit IgG (negative control, one replicate) or anti-histone H1 (positive control, one replicate) immunofluorescence by LSC. Tissue microarrays minimize inter-slide variability and standardize tissue sampling by combining multiple tissues on the same slide (26). Figure 3A shows how the percentage of MeCP2-negative cells was determined for each tissue sample on the microarray based on the appropriate Mecp2 2/y negative control. As the large dynamic range of MeCP2 expression limited the resolution of MeCP2negative cells from the MeCP2lo population, saturating LSC settings were used to precisely determine the percentage of MeCP2-negative cells (Fig. 3A, top four histograms). Two distinct peaks could be observed only in Mecp2 2/þ samples, representing MeCP2-negative (Mecp2 mutant-expressing) and MeCP2-positive (Mecp2 wt-expressing) cells. The gate for MeCP2-negative cells (region 1, colored in gray) was set to the right half maximum of the Mecp2 tm1.1Bird/y histogram (top left) and was used to determine the percentage of MeCP2-negative cells in each of the Mecp2 tm1.1Bird/þ tissues (as shown in the middle left histogram) in four replicate experiments (mean + SEM graphed in Fig. 4A and B, y-axes). The mean percentage of MeCP2-negative cells ranged from 20 to 39% for the five Mecp2 tm1.1Bird/þ mice, somewhat lower than the overall expected 50%. In contrast to the Mecp2 tm1.1Bird/y tissues that were negative for MeCP2 immunofluorescence, the Mecp2 tm1.1Jae//y mice had a background immunoreactivity (Fig. 3A, top right panel, filled histogram) that was higher than the IgG control Human Molecular Genetics, 2004, Vol. 13, No. 12 1281 Figure 4. XCI ratios influence the MeCP2 expression in Mecp2 wt-expressing cells of mosaic female mice. (A and B) Scattergrams plotting the percentage of MeCP2-negative population (mean + SEM of four replicates) on the y-axis versus the MeCP2 max pixel (A) or mean intergral/nuclear area (B) values (mean + SEM of four replicates) of the gated MeCP2-positive population for each mouse sample on the tissue microarray. Mecp2 þ/þ mice (open squares) were analyzed identically to the 10 Mecp2 2/þ mouse samples (closed circles) and show higher MeCP2 expression by both measurements. An inverse correlation was observed between the percentage of MeCP2-negative cells and MeCP2 expression in the wt-expressing cells of Mecp2 2/þ mice (P , 0.05 by F-test for both A and B). (C) Fluorescent micrographs of MeCP2 immunofluorescence showing absence of staining in nuclei from Mecp2tm1.1Bird/y cerebral tissue, reduced or absent expression in Mecp2tm1.1Bird/þ cerebral nuclei, and normal MeCP2 expression in Mecp2 þ/þ control tissue. Images were captured using identical exposure and deconvolution settings. Examples of the MeCP2-negative population (closed arrows), MeCP2lo nuclei (open arrows), and MeCP2hi nuclei (diamond arrows) are shown. Although the Mecp2tm1.1Bird/þ brain tissue shows a majority of MeCP2-positive (wt-expressing) nuclei, they are primarily of the MeCP2lo phenotype. (overlay). A similar background immunoreactivity can be explained as a truncated mutant protein by immunoblot for the Mecp2 tm1.1Jae/y but not for the Mecp2 tm1.1Bird/y mice (data not shown) and has been described previously (27). The gate for region 1 (gray, MeCP2-negative cells) was therefore set at the right half max of the saturated Mecp2 tm1.1Jae/y control (top right histogram), which also separated the lowest peak in the saturated Mecp2 tm1.1Jae/þ samples (middle right panel) and was used to determine the percentage of MeCP2-negative cells in the four replicate experiments (mean + SEM graphed in Fig. 4A and B, y-axes). The mean percentage of MeCP2-negative cells ranged from 18 to 37% for the five Mecp2 tm1.1Jae/þ mice. The overall mean for the 10 Mecp2 2/þ mice on the tissue microarray was 29.9 + 7.5%, which was significantly lower than the expected 50% by t-test (P , 0.0001). These results demonstrate unbalanced XCI patterns in favor of inactivating the mutant allele. The mean percentage of MeCP2-negative calculation obtained for each tissue was then used to set the gates for the histograms of MeCP2 fluorescence obtained at non-saturating LSC settings to obtain quantitative measurements of MeCP2 in the Mecp2 wt-expressing cells (MeCP2-positive) of each tissue, as shown in the bottom panel of Figure 3A (MeCP2-negative colored in gray, MeCP2-positive colored 1282 Human Molecular Genetics, 2004, Vol. 13, No. 12 in black). The values of MeCP2 max pixel (Fig. 4A) and MeCP2 integral/nuclear area (Fig. 4B) were obtained for the gated MeCP2-positive population for each tissue (x-axes, mean + SEM from four replicates). For both mouse models, the Mecp2 wt-expressing cells (MeCP2-positive) from the Mecp2 2/þ tissues (filled circles) showed lower MeCP2 expression than the equivalently gated MeCP2-positive cells from Mecp2 þ/þ tissues (open squares). The percentage of MeCP2-negative cells in the Mecp2 2/þ tissues inversely correlated with both the MeCP2 max pixel (P , 0.05 by F-test) and the MeCP2 integral/nuclear area (P , 0.05, by F-test) of the MeCP2-positive cells in the same tissue samples. In contrast, the analysis of histone H1 values in the MeCP2-positive cells showed no significant correlation with the percentage of MeCP2-negative cells by F-test (data not shown). Representative images of MeCP2 immunofluorescence are shown in Figure 4C, demonstrating the low MeCP2 expression characteristic of the MeCP2-positive nuclei from Mecp2 2/þ brain tissue compared with Mecp2 þ/þ controls. These results demonstrate that XCI ratios in Mecp2 2/þ mice can influence the wt Mecp2 expression. Human RTT brain samples also show an effect of XCI on wt MECP2 expression To investigate whether a similar effect of mosaic MECP2 mutation on MeCP2 expression in wt-expressing neurons could be observed in human female RTT brain samples, we created a human post-mortem brain tissue microarray containing six replicate cores sampled from frontal cerebral cortex of three patients with known MECP2 mutations and triplicate samples of control subjects of various ages. RT 1238 was from a previously described male subject with a MECP2 1154(del32) mutation that died at 21 months of age (28). The MeCP2 protein from this patient was predicted to lack 100 amino acids at the C terminus, including the C-terminal antibody epitope, and therefore this hemizygous male was used as the control for determination of the MeCP2-negative population. Figure 5A demonstrates that when the tissue microarray stained with anti-MeCP2 was scanned using saturating LSC settings for long red max pixel, the RT 1238 gated histogram (filled, top) overlapped with the non-specific antibody control (black overlay, top). Region 1 (gray, MeCP2negative population) was set to the right half maximum of the RT 1238 histogram and was used to determine the percentage of MeCP2-negative cells in two RTT female samples with MECP2 R168X truncating mutations at the same saturated settings (middle histograms). RT 3393 and RT 4312 showed 67 and 30% MeCP2-negative cells, respectively. These percentages were used to set region 1 under unsaturated LSC setting (lower histograms), so that the MeCP2-positive cells (region 2, black) could be analyzed for MeCP2 max pixel and intergral/nuclear area values. The same analysis was performed on samples from a group of six controls ranging from 11 to 30 years of age. Figure 5B demonstrates that while MeCP2-positive cells from both RT 3393 and RT 4312 had significantly lower MeCP2 max pixel values than controls, the difference was much more significant in RT 3393 than in RT 4312 (P , 0.001 and P , 0.05, respectively). For the MeCP2 integral/nuclear area measurement (Fig. 5C), MeCP2-positive cells from RT 3393, but not from RT 4312, were significantly different from controls (P , 0.01). In contrast, similar measurements of histone H1 were not significantly different between MeCP2-positive cells of patients and controls (Fig. 5B and C). These results from human RTT samples are therefore in agreement with the findings from Mecp2 2/þ mice in showing a non-cell-autonomous effect of MECP2/Mecp2 mutation on wt MECP2 expression that correlates with the percentage of MeCP2 mutant-expressing cells. Interestingly, the phenotype of RT 3393 (diagnosis at 4 months, death at 12 years) appeared to be more severe than that of RT 4312 (diagnosis at 18 months, death at 24 years). These results suggest that XCI can influence the RTT phenotype by both cell-autonomous and non-autonomous mechanisms. DISCUSSION In this report, we have used the quantitative method of detecting MeCP2 immunofluorescence by LSC in order to understand the normal developmental and regional expression of MeCP2 and to separately analyze MeCP2 mutant- and wt.expressing cells in human RTT samples and the Mecp2 2/þ mouse model of RTT. These analyses have allowed several novel observations to be made relevant for understanding the pathogenesis of RTT. First, we demonstrate that MeCP2 expression and localization is dynamically controlled at several stages during mammalian brain development. A timeline is shown in Figure 6A to summarize the developmental changes in MeCP2 and the phenotypic manifestations in the RTT mouse model. Although MeCP2 is expressed in virtually all murine cells and developmental stages, the level rises sharply in the medulla and thalamus around birth, then falls sharply by the first postnatal week. Between 1 and 3 weeks of age, MeCP2 immunofluorescence intensity rises sharply due to increased localization to multiple heterochromatic nuclear foci, characteristic of the MeCP2lo neurons. The phenotype of the Mecp2 2/y mice becomes apparent soon after, from around 3 to 6 weeks of age (14,15), suggesting that MeCP2 has an essential function at this developmental stage. The next change occurs in MeCP2 expression level in individual neurons throughout all brain regions, resulting in the gradual accumulation of the MeCP2hi population. The delay of a noticeable phenotype in the Mecp2 2/þ mice until adulthood (4 – 6 months) could be due to this continued increase in the MeCP2hi population throughout adulthood and the specific requirement for MeCP2 in this population. Although the developmental expression of MeCP2 has been described previously in several mammals (9,17 –20), our study of wt mice more completely investigates the changes at the single-cell level throughout the brain and at 20 different ages and identifies more dynamic changes in MeCP2 than were realized previously. Prior investigations performed by immunohistochemistry have identified more intensely stained neuronal nuclei at later postnatal ages, but these were not quantitated previously (9,18,20). Prior immunoblot analyses of olfactory bulb (17), cerebellum (20) and hippocampus (29), but not whole brain extracts (9), identified increased MeCP2 expression with postnatal age, consistent with our Human Molecular Genetics, 2004, Vol. 13, No. 12 1283 Figure 5. Analysis of RTT females with MECP2 mutations for evidence of an effect of XCI on wt MECP2 expression. (A) The LSC analysis of cerebral samples on a human tissue array containing samples from one male and two females with MECP2 truncation mutations analyzed at saturating (top and middle panels) and non-saturating (bottom) conditions. A two-step analysis was performed as for the mouse tissues in Figure 3A, to determine the percentage MeCP2-negative for each female sample, and then determine the MeCP2 and histone H1 expression of the MeCP2-positive cells in the two RTT female samples. (B) MeCP2 mean max pixel values of the MeCP2-positive cells were significantly lower compared with those of age-matched controls, although the significance was higher for sample RT 3393 with 67% MeCP2-negative cells compared with RT 4312 with only 30%. (C) MeCP2 mean integral/nuclear area measurements were significantly lower than controls for the MeCP2-positive population of the RT 3393 sample, but not the RT 4312 sample. P , 0.05; P , 0.01; P , 0.001. results showing an increased MeCP2hi population in these regions (Fig. 1). Our immunoblot analyses of MeCP2 in whole brain extracts (Fig. 2C and D) also failed to show MeCP2 expression increase in mouse adulthood, presumably because the individual subpopulations or brain regions were not fractionated. Another possibility could be that immunofluorescence-based assays detect changes in epitope exposure rather than expression changes. We believe this to be an unlikely explanation because MECP2 transcripts are also significantly increased in the MeCP2hi population (30) and immunoblots of specific brain regions also demonstrate postnatal increases in MeCP2 reactivity (17,20,29). In addition, our protocol involves boiling tissue sections for 1 h in antigen retrieval solution. Changes to the pH and citrate concentrations of the antigen retrieval solution do not significantly alter MeCP2 immunofluorescence (data not shown), suggesting that protein – protein interactions are unlikely to affect antibody binding in our assay. Two recent reports have described a second isoform of MeCP2 arising from alternative splicing of exon 2 and resulting in a change in the N terminus that is indistinguishable from the previously described isoform by immunoblot (31,32). As our study utilized a C-terminal reactive antibody for MeCP2, both MeCP2 isoforms were detected most likely by immunofluorescence and immunoblot. Future studies using isoform-specific reagents would be useful in determining if the dynamic MeCP2 expression changes are unique to the MeCP2B (MeCP2a in mouse) isoform predominant in brain (31,32). By examining MeCP2 expression levels in the wt-expressing cells of Mecp2 2/þ mosaic females we have also made several significant observations regarding the role of XCI on the pathogenesis of RTT. We demonstrate that the range of MeCP2 mutant-expressing cells from adult Mecp2 2/þ mice from two different mouse strains was 18 – 39% (mean 29.9%), significantly lower than the expected 50%. These results demonstrate an unbalanced XCI patterns in adult Mecp2 2/þ mice, favoring expression of the wt allele. Our results confirm those of a recent study examining XCI in the 1284 Human Molecular Genetics, 2004, Vol. 13, No. 12 Figure 6. Models for effect of developmental dynamics of MeCP2 expression and localization and the role of XCI on the pathogenesis of RTT. (A) Developmental timeline of MeCP2 expression and localization changes in the wt mouse and the development of the RTT-like phenotype in the Mecp2-null mouse model. (B) Model for the role of a non-cell-autonomous mechanism involved in the maturation of neurons from the MeCP2lo to MeCP2hi phenotype. In Mecp2 þ/þ mice, immature MeCP2lo neurons receive signals from surrounding neurons (arrows) that trigger them to become mature MeCP2hi neurons. In mosaic Mecp2 2/þ and RTT females, however, the Mecp2/ MECP2 mutant cells surrounding the wt-expressing neurons limit the maturational signals, so fewer wt neurons mature to the MeCP2hi phenotype. The higher the percentage of Mecp2 mutant-expressing cells in the brain, the greater the influence of this ‘bad neighborhood’ effect. Mecp2 308/þ mouse model (25). The selection against Mecp2 mutant-expressing cells could occur at the level of XCI in the early embryo, but a more likely explanation is a positive selection of the wt-expressing cells compared with themutant-expressing cells, similar to that observed in lymphocyte cultures from RTT blood (33). As many questions remain with regard to the timing and occurrence of the XCI bias in Mecp2 2/þ mice, future experiments examining XCI ratios in different mouse strains, tissues and developmental stages are warranted. Our most interesting and novel observation from these studies is that Mecp2 mutant-expressing cells influence the phenotype of the wt-expressing cells in Mecp2 2/þ female mice by a non-cell-autonomous mechanism. The data presented in Figure 4 demonstrate that the percentage of MeCP2-negative cells inversely correlates with MeCP2 expression in the MeCP2-positive cells. These data demonstrate that cell extrinsic factors influence the complex developmental control of MeCP2 expression during postnatal brain development. A model for this hypothesis is shown in Figure 6B. In wt Mecp2 þ/þ mice, immature neurons of the MeCP2lo phenotype would receive synaptic stimuli from surrounding neurons that cause them to undergo a maturational differentiation to become MeCP2hi neurons. In the Mecp2 2/þ brain, however, the wt-expressing neurons are in a ‘bad neighborhood’ being surrounded by neurons lacking functional MeCP2. They would therefore receive fewer extrinsic stimuli, resulting in fewer neurons progressing to the MeCP2hi phenotype and resulting in lower overall MeCP2 expression. Brains containing fewer MeCP2 mutant-expressing neurons would therefore be the least affected. Our recent evidence for multiple pathways affecting the level of MeCP2 expression in several neurodevelopmental disorders is also supportive of this model (30). Two recent studies demonstrating brain-derived neurotrophic factor (BDNF) as a potential neuron-specific target for MeCP2 may be relevant to our model, as BDNF is a soluble factor that promotes survival and maturation of neurons (27,34). Cortical neurons from Mecp2-null mice had higher levels of BDNF than wt control neurons prior to stimulation, but not following KCl-mediated activation, suggesting that MeCP2 is involved in the repression of BDNF prior to neuronal activation (27). Although increased levels of BDNF may be expected to promote rather than prevent neuronal maturation, the direct role of BDNF and other potential gene targets of MeCP2 in the pathogenesis of RTT remain to be investigated. Two brain samples from female RTT patients with MECP2 mutations also showed evidence for a non-cell-autonomous mechanism influencing MeCP2 expression in wt-expressing cells. The most striking evidence was from RT 3393, showing 67% MECP2 mutant-expressing cells, higher than that observed in any of the Mecp2 2/þ mice. The discordance between random XCI patterns generally found in RTT patient brain and blood compared with the non-random patterns observed in Mecp2 2/þ mice could be due to a sampling bias in the human population towards those that meet the diagnostic criteria for RTT (25). Perhaps MECP2 mutations could be found in additional females with milder forms of mental retardation and favorable XCI ratios. Finally, our data may have implications for the potential treatment of RTT patients if the cell extrinsic factors regulating the level of MeCP2 expression in wt-expressing neurons could be characterized and mimicked pharmacologically. MATERIALS AND METHODS Mouse tissue Mecp2 tm1.1Bird/þ (Jackson Laboratories) and Mecp2 tm1.1Jae/þ (MMRRC) were mated with wt C57BL/6J mice (Jackson Laboratories) to produce the four possible genotypes. Genotyping was performed using protocols provided by the commercial vendors. An additional multiplex PCR was performed with genomic DNA to confirm the presence or absence of Mecp2 exon 4 in Mecp2 tm1.1Bird/þ mice. PCR conditions were as follows: 0.02 mM of primer pair 105 (50 -GTGAAGGAGTC TTCCATACGGTC-30 ) and 106 (50 -TCTCCTTGCTTTTAC GCCC-30 , 183 bp product) and 0.4 mM of primer pair 107 Human Molecular Genetics, 2004, Vol. 13, No. 12 (50 -ATGGCCTGCCAGAGTCGT-30 ) and 108 (50 -CAATTGGAACTGTCAACTACGGTT-30 , 618 bp product) with PCR amplification performed through 33 cycles of 35 s at 958C, 45 s at 59.28C and 1 min at 728C. Mice were euthanized at different developmental time points (P1 corresponds to day of birth). Whole brain specimens were cut sagittaly at the midline using a sterile scalpel and one half was placed in 10% neutral buffered formalin for 2 –3 days and then embedded in paraffin blocks; the other half was placed in Trizol and stored at 2808C for protein and nucleic acid extractions. Sections of 1 mm were removed from each block followed by sectioning at 5 mm for experimental slides. The H&E slides were prepared for each brain specimen to aid in identification of brain regions. Epitope exposure was carried out as described previously (8). Tissue microarray construction Triplicate 600 mm cores of cerebral cortex were removed from five Mecp2 tm1.1Bird/þ, five Mecp2 tm1.1Jae/þ and three Mecp2 þ/þ controls (P20 –27w); one Mecp2 tm1.1Bird/y,and one Mecp2 þ/y littermate (P10w); one Mecp2 tm1.1Jae/y and one Mecp2 þ/y littermate control (P10w) paraffin ‘donor’ blocks based on H&E slide reference and inserted into a recipient paraffin block using a manual tissue arrayer (Beecher Instruments). Resultant blocks were incubated at 508C for 10 min to set the cores and serial 5 mm sections were cut from the array blocks and stained as above for immunofluorescence. An H&E slide was made to verify tissue sampling. A human developmental tissue microarray described previously (10), was supplemented with six cerebral cortex samples from three RTT patients and processed as above. Use of human samples was approved by the institutional IRB (#200210814-1). Immunofluorescence Anti-MeCP2 C terminus (Affinity Bioreagents), mouse IgG, anti-histone H1 (Upstate Biotechnology) were diluted 1 : 100 in IF stain buffer [phosphate buffered saline (PBS) 1% fetal calf serum/0.5% Tween-20] and incubated on slides for 2 h at 378C, followed by three washes in PBS/0.5% Tween-20. Cy5-labeled secondary antibody to rabbit IgG (Jackson Immunoresearch) or Cascade Blue-labeled secondary antibody to mouse IgG (Molecular Probes) were diluted 1 : 100 in IF buffer containing 10 mg/ml Sytox Green (Molecular Probes) and incubated on slides for 1 h at 378C, followed by three washes in PBS/0.5% Tween-20. Slides were mounted in 50% glycerol with 10 mg/ml Sytox Green and sealed with nail polish. LSC and fluorescence microscopy Slides stained with anti-MeCP2, anti-histone H1 (positive control) or IgG negative control were scanned on an LSC-1 under the 20 objective using appropriate settings (CompuCyte, Cambridge, MA, USA). Cells were contoured on the basis of Sytox Green nuclear fluorescence, optimized for each experimental and control slide pair to include as many individually contoured cells as possible. Cell clusters were gated out of analysis as described previously (8). Slides were visualized 1285 using a 60 oil immersion lens on a Zeiss Axioplan II microscope and images collected using digital deconvolution software as described previously (8). Immunoblotting Protein was isolated from mouse brain using TRIzol (Invitrogen) according to manufacturers protocol. An amount of 5 mg of protein was loaded into each lane in pre-cast 4– 15% Tris – HCl polyacrylamide gels (Biorad), and 10 ml of Kaleidoscope pre-stained standards (Biorad) were loaded as a molecular weight marker. Gels were electrophoresed and transferred to nitrocellulose membranes. Membranes were washed with tris-buffered saline (TBS) (Biorad) and blocked with 5% non-fat dry milk (Carnation) in TBS. Primary antibody incubation with anti-MeCP2 (Affinity) diluted 1: 2000 in TBS was done overnight at 48C. Blots were then washed in TBS and incubated with a 1 : 2500 dilution of goat anti-rabbit horseradish peroxidase (HRP) (Biorad) for 40 min at room temperature. After washing in TBS, Supersignal West Pico chemiluminescent substrate (Pierce) was added, and the results recorded on photographic film. Glyceraldehyde 3-phosphate dehydrogenase (Advanced Immunochemical) was diluted 1 : 600, then detected with goat anti-mouse HRP (Biorad) in a 1 : 10,000 dilution, as above. ACKNOWLEDGEMENTS The authors thank M. Lalande for critical review of the manuscript and W.P. Robinson for statistical advice. This work was supported in part by the NIH (R01HD/NS41462), the Rett Syndrome Research Foundation, and the U.C. Davis M.I.N.D. Institute. Mecp2 tm1.1Jae mice were obtained from the Mouse Mutant Regional Resource Center at U.C. Davis (supported in part by NIH U42 RR14905). Human tissue samples were obtained from the University of Maryland Brain and Tissue Bank for Developmental Disorders (supported by NIH N01-HD-1-3138) and the Harvard Brain Tissue Resource Center (supported in part by PHS MH/NS 31862). REFERENCES 1. Van den Veyver, I.B. and Zoghbi, H.Y. (2000) Methyl-CpG-binding protein 2 mutations in Rett syndrome. Curr. Opin. Genet. Dev., 10, 275–279. 2. Shahbazian, M.D. and Zoghbi, H.Y. (2002) Rett syndrome and MeCP2: linking epigenetics and neuronal function. Am. J. Hum. Genet., 71, 1259–1272. 3. Armstrong, D.D. (1997) Review of Rett syndrome. J. Neuropathol. Exp. Neurol., 56, 843 –849. 4. Amir, R.E., Van den Veyver, I.B., Wan, M., Tran, C.Q., Francke, U. and Zoghbi, H.Y. (1999) Rett syndrome is caused by mutations in X-linked MECP2, encoding methyl-CpG-binding protein 2. Nat. Genet., 23, 185 –188. 5. Coy, J.F., Sedlacek, Z., Bachner, D., Delius, H. and Poustka, A. (1999) A complex pattern of evolutionary conservation and alternative polyadenylation within the long 30 -untranslated region of the methylCpG-binding protein 2 gene (MeCP2) suggests a regulatory role in gene expression. Hum. Mol. Genet., 8, 1253–1262. 6. D’Esposito, M., Quaderi, N.A., Ciccodicola, A., Bruni, P., Esposito, T., D’Urso, M. and Brown, S.D. (1996) Isolation, physical mapping, and northern analysis of the X-linked human gene encoding methyl CpG-binding protein, MECP2. Mamm. Genome, 7, 533–535. 1286 Human Molecular Genetics, 2004, Vol. 13, No. 12 7. Reichwald, K., Thiesen, J., Wiehe, T., Weitzel, J., Poustka, W.A., Rosenthal, A., Platzer, M., Stratling, W.H. and Kioschis, P. (2000) Comparative sequence analysis of the MECP2-locus in human and mouse reveals new transcribed regions. Mamm. Genome, 11, 182–190. 8. LaSalle, J., Goldstine, J., Balmer, D. and Greco, C. (2001) Quantitative localization of heterologous methyl-CpG-binding protein 2 (MeCP2) expression phenotypes in normal and Rett syndrome brain by laser scanning cytometry. Hum. Mol. Genet., 10, 1729– 1740. 9. Shahbazian, M.D., Antalffy, B., Armstrong, D.L. and Zoghbi, H.Y. (2002) Insight into Rett syndrome: MeCP2 levels display tissue- and cell-specific differences and correlate with neuronal maturation. Hum. Mol. Genet., 11, 115 –124. 10. Balmer, D., Goldstine, J., Rao, Y.M. and LaSalle, J.M. (2003) Elevated methyl-CpG-binding protein 2 expression is acquired during postnatal human brain development and is correlated with alternative polyadenylation. J. Mol. Med., 81, 61–68. 11. Lewis, J.D., Meehan, R.R., Henzel, W.J., Maurer-Fogy, I., Jeppesen, P., Klein, F. and Bird, A. (1992) Purification, sequence, and cellular localization of a novel chromosomal protein that binds to methylated DNA. Cell, 69, 905–914. 12. Nan, X., Tate, P., Li, E. and Bird, A. (1996) DNA methylation specifies chromosomal localization of MeCP2. Mol. Cell. Biol., 16, 414–421. 13. Nan, X., Campoy, F.J. and Bird, A. (1997) MeCP2 is a transcriptional repressor with abundant binding sites in genomic chromatin. Cell, 88, 471 –481. 14. Chen, R.Z., Akbarian, S., Tudor, M. and Jaenisch, R. (2001) Deficiency of methyl-CpG binding protein-2 in CNS neurons results in a Rett-like phenotype in mice. Nat. Genet., 27, 327 –331. 15. Guy, J., Hendrich, B., Holmes, M., Martin, J.E. and Bird, A. (2001) A mouse Mecp2-null mutation causes neurological symptoms that mimic Rett syndrome. Nat. Genet., 27, 322 –326. 16. Shahbazian, M., Young, J., Yuva-Paylor, L., Spencer, C., Antalffy, B., Noebels, J., Armstrong, D., Paylor, R. and Zoghbi, H. (2002) Mice with truncated MeCP2 recapitulate many Rett syndrome features and display hyperacetylation of histone H3. Neuron, 35, 243–254. 17. Cohen, D.R., Matarazzo, V., Palmer, A.M., Tu, Y., Jeon, O.H., Pevsner, J. and Ronnett, G.V. (2003) Expression of MeCP2 in olfactory receptor neurons is developmentally regulated and occurs before synaptogenesis. Mol. Cell. Neurosci., 22, 417 –429. 18. Akbarian, S., Chen, R.Z., Gribnau, J., Rasmussen, T.P., Fong, H., Jaenisch, R. and Jones, E.G. (2001) Expression pattern of the Rett syndrome gene MeCP2 in primate prefrontal cortex. Neurobiol. Dis., 8, 784 –791. 19. Jung, B.P., Jugloff, D.G., Zhang, G., Logan, R., Brown, S. and Eubanks, J.H. (2003) The expression of methyl CpG binding factor MeCP2 correlates with cellular differentiation in the developing rat brain and in cultured cells. J. Neurobiol., 55, 86 –96. 20. Mullaney, B.C., Johnston, M.V. and Blue, M.E. (2004) Developmental expression of methyl-CpG binding protein 2 is dynamically regulated in the rodent brain. Neuroscience, 123, 939–949. 21. Zoghbi, H.Y., Percy, A.K., Schultz, R.J. and Fill, C. (1990) Patterns of X chromosome inactivation in the Rett syndrome. Brain Dev., 12, 131–135. 22. Amir, R.E., Van den Veyver, I.B., Schultz, R., Malicki, D.M., Tran, C.Q., Dahle, E.J., Philippi, A., Timar, L., Percy, A.K., Motil, K.J. et al. (2000) Influence of mutation type and X chromosome inactivation on Rett syndrome phenotypes. Ann. Neurol., 47, 670 –679. 23. Shahbazian, M.D., Sun, Y. and Zoghbi, H.Y. (2002) Balanced X chromosome inactivation patterns in the Rett syndrome brain. Am. J. Med. Genet., 111, 164–168. 24. Wan, M., Lee, S.S., Zhang, X., Houwink-Manville, I., Song, H.R., Amir, R.E., Budden, S., Naidu, S., Pereira, J.L., Lo, I.F. et al. (1999) Rett syndrome and beyond: recurrent spontaneous and familial MECP2 mutations at CpG hotspots. Am. J. Hum. Genet., 65, 1520–1529. 25. Young, J.I. and Zoghbi, H.Y. (2004) X-chromosome inactivation patterns are unbalanced and affect the phenotypic outcome in a mouse model of Rett syndrome. Am. J. Hum. Genet., 74, 511–520. 26. Kallioniemi, O.P., Wagner, U., Kononen, J. and Sauter, G. (2001) Tissue microarray technology for high-throughput molecular profiling of cancer. Hum. Mol. Genet., 10, 657– 662. 27. Chen, W.G., Chang, Q., Lin, Y., Meissner, A., West, A.E., Griffith, E.C., Jaenisch, R. and Greenberg, M.E. (2003) Derepression of BDNF transcription involves calcium-dependent phosphorylation of MeCP2. Science, 302, 885 –889. 28. Hoffbuhr, K., Devaney, J.M., LaFleur, B., Sirianni, N., Scacheri, C., Giron, J., Schuette, J., Innis, J., Marino, M., Philippart, M. et al. (2001) MeCP2 mutations in children with and without the phenotype of Rett syndrome. Neurology, 56, 1486–1495. 29. Cassel, S., Revel, M.O., Kelche, C. and Zwiller, J. (2004) Expression of the methyl-CpG-binding protein MeCP2 in rat brain. An ontogenetic study. Neurobiol. Dis., 15, 206–211. 30. Samaco, R.C., Nagarajan, R.P., Braunschweig, D. and LaSalle, J.M. (2004) Multiple pathways regulate MeCP2 expression in normal brain development and exhibit defects in autism-spectrum disorders. Hum. Mol. Genet., 13, 629 –639. 31. Kriaucionis, S. and Bird, A. (2004) The major form of MeCP2 has a novel N-terminus generated by alternative splicing. Nucl. Acids Res. 32, 1818–1823. 32. Mnatzakanian, G.N., Lohi, H., Munteanu, I., Alfred, S.E., Yamada, T., MacLeod, P.J., Jones, J.R., Scherer, S.W., Schanen, N.C., Friez, M.J. et al. (2004) A previously unidentified MECP2 open reading frame defines a new protein isoform relevant to Rett syndrome. Nat. Genet., 36, 339 –341. 33. Balmer, D., Arredondo, J., Samaco, R.C. and LaSalle, J.M. (2002) MECP2 mutations in Rett syndrome adversely affect lymphocyte growth, but do not affect imprinted gene expression in blood or brain. Hum. Genet., 110, 545– 552. 34. Martinowich, K., Hattori, D., Wu, H., Fouse, S., He, F., Hu, Y., Fan, G. and Sun, Y.E. (2003) DNA methylation-related chromatin remodeling in activity-dependent BDNF gene regulation. Science, 302, 890 –893.