* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download Hypoplastic left heart syndrome: From in
Electrocardiography wikipedia , lookup
Heart failure wikipedia , lookup
Cardiothoracic surgery wikipedia , lookup
Management of acute coronary syndrome wikipedia , lookup
Hypertrophic cardiomyopathy wikipedia , lookup
Coronary artery disease wikipedia , lookup
Myocardial infarction wikipedia , lookup
Arrhythmogenic right ventricular dysplasia wikipedia , lookup
Mitral insufficiency wikipedia , lookup
Cardiac surgery wikipedia , lookup
Quantium Medical Cardiac Output wikipedia , lookup
Lutembacher's syndrome wikipedia , lookup
Atrial septal defect wikipedia , lookup
Dextro-Transposition of the great arteries wikipedia , lookup
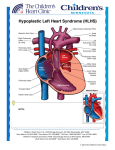
Seminars in Fetal & Neonatal Medicine (2005) 10, 553e566 www.elsevierhealth.com/journals/siny Hypoplastic left heart syndrome: From in-utero diagnosis to school age Jack Rychik a,b,* a b Fetal Heart Program, The Cardiac Center at The Children’s Hospital of Philadelphia, PA, USA University of Pennsylvania School of Medicine, Philadelphia, PA, USA KEYWORDS Hypoplastic left heart syndrome; Congenital heart disease; Fetal diagnosis Summary HLHS can be treated with successful survival outcome. Prenatal diagnosis of the anomaly is now quite common. Our understanding of the developmental aspects of HLHS during the second and third trimesters of gestation is advancing. Survivors of surgery are being closely followed and studied as they proceed forwards in time. A number of morbidities are identified. Many questions concerning the pathophysiological mechanisms of these morbidities exist. New therapies and treatments will certainly arise to meet the challenges these children face as they enter into adulthood, and as our understanding of this unique cardiovascular state progresses. ª 2005 Elsevier Ltd. All rights reserved. Introduction In the current era, nearly all forms of congenital heart disease are amenable to highly successful reparative strategies. However, one of the most persistently difficult malformations to treat is when there is underdevelopment, or hypoplasia, of a ventricle. One such anomaly, the hypoplastic left heart syndrome (HLHS), is defined as an anatomical constellation in which the left-sided structures (mitral valve, left ventricle, aortic valve, aorta) are inadequate and non-viable for support of the systemic circulation. Without early neonatal surgical intervention, this anomaly is uniformly fatal. Since * Fetal Heart Program, The Children’s Hospital of Philadelphia, 34th Street and Civic Center Boulevard, Philadelphia, PA 19104, USA. Tel.: C1 215 590 2192; fax: C1 267 426 5082. E-mail address: [email protected] the early 1980s, a series of surgical interventions have been developed, and have subsequently evolved, that allow for reconstruction of the circulation and survival.1e3 However, despite major strides in improving survival, surgical mortality remains at least 5e10% in the best of series with ongoing life-long morbidity. This article will review the advances made in diagnosis and management of HLHS in the past two decades, with emphasis on the progress made in understanding the fetal aspects of this anomaly, recent advances in surgical strategy, and some of the long-term complications that these children face as they grow into adulthood. Prenatal diagnosis and perinatal course HLHS occurs in approximately 200e300 per million livebirths.4 The anatomy typically consists of 1744-165X/$ - see front matter ª 2005 Elsevier Ltd. All rights reserved. doi:10.1016/j.siny.2005.08.006 554 hypoplasia of the left ventricle with mitral atresia or stenosis, aortic atresia or stenosis, and hypoplasia of the ascending aorta. As fetal ultrasound techniques and obstetric ultrasound operator skill have improved over the past decade, prenatal detection rates for HLHS have increased substantially. The diagnosis is now commonly made prior to birth as it can be determined from simple absence of the normal ‘four-chamber’ heart. HLHS can be associated with chromosomal anomalies, most commonly Turner’s syndrome (45, XO),5 although the majority of patients do not have any identifiable chromosomal or genetic association. Extracardiac anomalies can also be associated and have been described in up to 28% of cases.6 The recurrence rate for bearing another child with left-sided heart disease (e.g. bicuspid aortic valve, coarctation of the aorta) after having one child with HLHS is quite high compared with the recurrence rate for other forms of heart disease. Recurrence of left-sided congenital heart disease (CHD) is reported to be anywhere from 2% to 13%.7 This lends credence to the notion of a strong genetic component or familial predisposition to the disease. Specific gene loci have, however, not yet been identified. Multiple genotypic configurations are likely to contribute to the heterogeneous group of phenotypic anatomical configurations called ‘HLHS’. Features of in-utero diagnosis HLHS is perceived to be a clinically silent disease while in utero. Absence of a viable left ventricle leads to dilation and hypertrophy of the right ventricle. The right ventricle experiences an increase in volume load, as blood is diverted away from the underdeveloped left side. Both the pulmonary and systemic vasculatures are perfused via the right ventricle and pulmonary artery, with patency of the ductus arteriosus assuring blood supply to the systemic circulation. Most fetuses with HLHS are asymptomatic prior to birth and come to gestational term without difficulty. Hydrops fetalis or fetal demise is unusual; when present, it is commonly due to causes other than heart failure. This suggests little physiological disturbance of the cardiovascular system while in utero, but a more careful analysis reveals otherwise. Fetuses with HLHS are typically small for gestational age.8 Whether this is a genetically associated finding or acquired during development, perhaps related to abnormal blood flow patterns, is unclear. Although the absence of hydrops in the typical case suggests adequate cardiac output, evidence has recently been found for diminished total cardiac output in the fetus with HLHS J. Rychik compared with control normal subjects with two ventricles (A. Szwast, unpublished data). In a series of 40 normal fetuses, combined cardiac output of both ventricles as measured by Doppler echocardiography was 508 G 88 ml/min/kg compared with cardiac output in an HLHS group of 18 fetuses of 419 G 122 ml/min/kg (P ! 0.05). This suggests incomplete right ventricular compensation for absence of the left ventricle in fetal HLHS, with approximately 20% diminution in total combined cardiac output as measured by Doppler techniques. The decrement in cardiac output may explain the limitations in somatic growth seen in these fetuses. As we shall see, additional flow disturbances distinguish the cardiovascular system of a fetus with HLHS from a fetus with a normal two-ventricle heart. Assessment of a fetus with HLHS via fetal echocardiography reveals normal umbilical arterial flow patterns, suggesting healthy placental function. When performing fetal echocardiography, diagnostic features to focus on include morphometric assessment of the left ventricle, mitral valve, aortic valve and ascending aorta. In cases of mitral atresia, the left ventricle may be completely absent. In cases of mitral stenosis, a small hypertrophied left ventricle can be seen. Echocardiographic brightness of the left ventricular endocardium suggests the presence of endocardial fibro-elastosis; a fibrotic change in the endocardial portion of the myocardium found in subjects with left-sided obstructive disease. Colour Doppler flow mapping may demonstrate a small amount of antegrade flow in cases of stenosis, or absence of flow across the mitral or aortic valves in cases of atresia. In the presence of aortic atresia, the ascending aorta may be quite miniscule, functioning as a common coronary artery with retrograde perfusion via the transverse aorta from the ductus arteriosus. The tricuspid valve may be abnormal in a fetus with HLHS, and may be incompetent. A mild degree of tricuspid regurgitation is common, but severe regurgitation can also be seen. In such cases, hydrops may develop as a consequence of diminished forward flow and elevated central venous pressure.9 Tricuspid regurgitation may progress during gestation; when severe, it may result in right ventricular dysfunction.10 In those that survive to term, severe tricuspid regurgitation is a risk factor for successful staged reconstruction. Fetal blood flow patterns in HLHS: pulmonary vasculature and restrictive atrial septum Doppler echocardiography has allowed in situ observation of blood flow patterns of fetuses Hypoplastic left heart syndrome with HLHS. Investigation of these blood flow patterns has resulted in a much clearer understanding of this complex and unique physiology. In fetal HLHS, placental and systemic venous return drains to the right atrium. Blood flows across the tricuspid valve into the right ventricle and is ejected into the pulmonary artery. The majority of blood volume is then delivered across the ductus arteriosus to the descending aorta, with retrograde perfusion of the transverse aorta, head vessels, ascending aorta and coronary arteries, depending upon the degree of competitive antegrade flow from the underdeveloped left ventricle. A portion of the blood volume delivered to the main pulmonary artery also perfuses the branch pulmonary arteries and the pulmonary vasculature. Elevated pulmonary vascular resistance limits the amount of pulmonary blood flow in the fetus. The precise fraction of pulmonary blood flow varies with gestational age, with an increasingly greater amount occurring later in gestation (up to 25% of the total cardiac output).11 Although the amount of pulmonary blood flow as a fraction of the total cardiac output in the fetus is relatively small, it is believed to be an important variable in promoting normal pulmonary vascular development. In a fetus with HLHS, pulmonary venous return is to a left atrium that may have no other egress except across the atrial septum, as the mitral valve and left ventricle are underdeveloped and may not allow for adequate left atrial decompression. Hence, the size of the interatrial communication is critical to determining the degree of left atrial decompression, and will determine the amount of forward pulmonary blood flow that will take place. In the presence of a large patent foramen ovale, or atria septal defect, left atrial decompression takes place and forward pulmonary blood flow will be normal. However, in conditions of a very restrictive or intact atrial septum, left atrial and pulmonary venous pressure will be elevated, and forward flow into the pulmonary arteries will be limited (Fig. 1). Blood will be preferentially shunted away from the lungs towards the ductus arteriosus and systemic circulation. Persistent pulmonary venous hypertension and limited pulmonary arterial blood flow can result in developmental and histological changes within the lung vasculature. While in utero, fetuses suffer no clinical symptoms in relation to a restrictive atrial septum as the lungs are not inflated and do not provide for oxygenation. However, at birth, with expansion of the lungs at the first breath, pulmonary venous return is immediately impaired leading to pulmonary venous congestion and severe hypoxaemia. Infants born with HLHS and an intact or restrictive 555 atrial septum constitute an urgent situation, and require procedures to open the atrial septum immediately. The outcome for these infants, despite timely and adequate opening of the atrial septum, is extremely poor.12 Prenatal identification of this phenomenon is possible by observation of the appearance of the atrial septum on twodimensional echocardiography, but also by analysis of pulmonary venous flow patterns obtained using Doppler echocardiography (Fig. 2).13 In a review of experience at The Children’s Hospital of Philadelphia, only 17% of newborns with intact atrial septum survived surgical reconstruction. Death is typically related to respiratory insufficiency and hypoxaemia. Autopsy analysis of the pulmonary vasculature in infants with HLHS and intact atrial septa reveals marked histological changes in the pulmonary veins with severe thickening and ‘arterialization’ (Fig. 3). These histological findings suggest the presence of a fixed pulmonary vascular anomaly in addition to the structural cardiac abnormality of HLHS.12 The advent of fetal echocardiography has allowed for reliable identification of HLHS with an intact atrial septum prior to birth. In order to reduce the amount of time after placental separation in which there is severe hypoxaemia, and in order to most effectively open the atrial septum, the author’s group has undertaken a strategy of Caesarean section delivery at term with immediate surgical resection of the atrial septum within the first hour of life. Two operating rooms are prepared adjacent to each other with a dedicated multidisciplinary team involved in the care of the mother and infant. In a series of four such cases, despite successful fetal delivery and atrial septal resection, none of the infants survived beyond 30 days of life (unpublished data). Other centres have reported some improvement in results with catheter-driven interventions performed immediately after birth; however, outcome is still poor in comparison with those with a naturally open atrial septum.14 Despite proper identification of the disease with prompt and immediate postnatal intervention, outcome remains extremely poor. Pulmonary vascular development has been deleteriously affected while in utero; as such, creating an open atrial septal communication after birth is too late to affect the process. The ability to identify patients with the disease reliably and the extremely poor postnatal outcome makes HLHS with intact/restrictive atrial septum an ideal lesion on which to attempt prenatal intervention. These techniques are currently being explored at a number of centres around the world.15 Beyond the technical challenge of performing an adequate prenatal 556 J. Rychik Figure 1 (A) Patterns of blood flow in the normal fetal heart. The left ventricle fills predominantly from the right to left shunting across the foramen ovale, but also with a small amount returning to the left atrium via the pulmonary veins. (B) Blood flow patterns in a fetus with hypoplastic left heart syndrome and an open atrial septum. Blood returning to the left atrium via the pulmonary veins cannot drain into the hypoplastic left ventricle, but finds its way across the atrial septum to the right atrium. The right atrium and right ventricle therefore receive the entire complement of venous return, both systemic and pulmonic. (C) Blood flow pattern in a fetus with an intact atrial septum. Pulmonary venous return enters the left atrium but has no egress. Left atrial hypertension and pulmonary venous hypertension ensues. Occasionally, blood will find its way out of the left atrium via primitive venous collaterals such as a levo-atrial cardinal vein. Ao, aorta; IVC, inferior vena cava; LA, left atrium; LV, left ventricle; PA, pulmonary artery; PDA, patent ductus arteriosus; RA, right atrium; RV, right ventricle; SVC, superior vena cava. opening of the atrial septum is the dilemma of timing and potential reversibility of the process. These questions are to be addressed via ongoing investigations in the near future. Cerebrovascular flow: the heartebrain interaction There is concern about the adequacy of blood volume delivered to the brain in a developing fetus with HLHS. In the normal fetus, flow is antegrade via the left ventricle and ascending aorta to the carotid arteries. The ascending aorta is a relatively large vessel, carrying approximately 40% of the combined cardiac output in the normal twoventricle state. However, in a fetus with HLHS, antegrade flow in the ascending aorta is very limited, or non-existent, and the ascending aorta is variably small. The cerebral circulation is supplied by the transverse aorta, which is perfused retrograde from the ductus arteriosus. Hence the blood volume delivered across the transverse aorta is only a small fraction of that which is ejected from the right ventricle. Although precise measures of cerebrovascular flow can be difficult, a measure of relative cerebrovascular resistance is possible. The pulsatility index (PI) is a Doppler-derived measure of downstream vascular resistance and can be calculated easily by analysis of the Doppler spectral waveform (Fig. 4). Cerebrovascular resistance, as measured by applying Doppler echocardiography in the middle cerebral artery, can be compared with placental vascular resistance, as measured in the umbilical artery. In the normal healthy state, placental vascular resistance is very low due to its highly vascular nature, while cerebrovascular resistance is relatively high. The PI ratio of middle cerebral artery to umbilical artery is high (O1). Under Hypoplastic left heart syndrome 557 Figure 2 Spectral display of fetal pulmonary vein Doppler flow patterns. In the top panel is an example of a fetus with hypoplastic left heart syndrome (HLHS) and an open atrial septum. The predominance of flow is forward out of the vein as designated by flow above the baseline. There is only a small amount of reversal of flow (below the baseline) with atrial contraction, designated by the line and cross-hair. In the bottom panel is an example of a fetus with HLHS and an intact atrial septum. Note the limited amount of forward flow (above the baseline) compared with the panel above. Also note the prominent reversal of flow (below the baseline) designated by the line and cross-hair. The peak velocity of reversed flow is equal to that of forward flow. This suggests physiological impairment to left atrial egress and is a marker for severe pulmonary vascular disease and the resulting clinical sequelae after birth. pathological conditions such as placental insufficiency or fetal distress (e.g. infection, hypovolaemia, heart failure), autoregulatory mechanisms come into play. This results in diminution of cerebrovascular resistance in order to preserve the volume of blood flow to the brain (Fig. 5). Hence, in a fetus in distress, the ratio of middle cerebral artery PI to umbilical artery PI reaches 1 or even !1. This phenomenon is called ‘cephalization’ in which blood volume, in response to regional changes in vascular resistance, is redistributed towards a vital organ, the brain. In HLHS, investigators have found a shift in resistance ratios compared with normal, with ‘cephalization’ a common finding.16 Kaltman et al. investigated the middle cerebral artery PIs of a large Figure 3 Histological stain of pulmonary veins in hypoplastic left heart syndrome. The panel on the left is an example of a pulmonary vein in a newborn with an open atrial septum. The panel on the right is an example of a pulmonary vein in a newborn with an intact atrial septum. Note the thickened vessel wall and the presence of multiple darkly stained elastic laminae, or ‘arterialization’ of the pulmonary vein. 558 J. Rychik Figure 4 Method used for calculation of the pulsatility index (PI). Doppler spectral example of a normal umbilical artery tracing. series of fetuses with both right- and left-sided obstructive lesions.17 Fetuses with right-sided obstructive disease had relatively high PIs compared with normal fetuses, suggesting elevated cerebrovascular resistance, while those with HLHS had relatively low PIs compared with normal fetuses and markedly lower PIs than fetuses with right-sided anomalies (Fig. 6). These findings can be logically explained as follows. In a fetus with a hypoplastic right ventricle or pulmonary atresia, blood flow is shunted towards the left side with an increased amount of blood volume filling the aorta and a concomitant increase in blood volume potentially being delivered to the brain. In an attempt to limit this increase in flow, autoregulatory mechanisms result in an increase in cerebrovascular resistance. In Figure 5 Doppler spectral display of flow in the middle cerebral artery. The top panel shows an example of normal high vascular resistance. This is suggested by the relatively low diastolic velocity (D) compared with the systolic velocity (S). The bottom panel is an example of abnormal low vascular resistance. This is suggested by the elevated diastolic velocity. Hypoplastic left heart syndrome 559 impaired head and brain growth. Further research in this area will lead to an improved understanding of the possible developmental links between HLHS and brain dysfunction, now commonly identified in this form of congenital heart disease (see below). Surgical concepts and results Figure 6 Pulsatility index (PI) z scores for gestational age for different groups of fetuses sampled. One hundred and twenty-five normal fetuses with two ventricles and no CHD were studied as controls, with a mean PI z score of nearly zero. Twenty-one fetuses with left-sided heart disease (LSOL), but with two ventricles and normal left ventricular output, also had normal PI z scores. However, 23 fetuses with right-sided heart disease (RSOL) had significantly higher mean PI z scores (P ! 0.05) and 34 fetuses with hypoplastic left heart syndrome (HLHS) had significantly lower mean PI z scores (P ! 0.05) than normal. In other words, fetuses with right-sided disease had higher middle cerebral artery vascular resistance than normal, and fetuses with HLHS had lower middle cerebral artery vascular resistance than normal. contradistinction, in a fetus with HLHS, blood flow is shunted away from the left side and is delivered to the transverse aorta in a retrograde fashion. A decreased complement of blood volume is delivered to the brain. Hence, in an attempt to increase cerebral blood flow, autoregulatory mechanisms promote cerebrovascular vasodilation, with a decrease in vascular resistance. These data suggest a very fundamental phenomenon not previously appreciated in fetuses with CHD. A dynamic interaction exists between the heart and the peripheral vascular beds. Type of CHD and the subsequent alterations in flow patterns as a consequence may result in changes in the peripheral vascular beds, with potential ramifications for organ perfusion. The implications of this phenomenon are profound as the presence of structural CHD may, in fact, exert the degree to which blood volume is delivered to the developing fetal organs. In particular, the relationship between fetal cerebral blood flow and neurocognitive outcome in children with HLHS is of great interest. Shillingford et al. investigated the relationship between newborn head circumference and size of ascending aorta in infants with HLHS.18 They found a striking relationship between small head circumference and small ascending aortic size, suggesting that early diminished forward flow in the aorta may lead to The surgical approach to reconstruction for HLHS is well established and performed regularly in many centres in North America and Europe. Initially conceived by William Norwood in the late 1970s,19 the approach has undergone modification and evolution resulting in dramatically improved survival statistics.2 Currently, the strategy consists of three operations (Table 1). The overall objective is to ultimately assign the right ventricle to the task of propelling blood into the systemic circulation, and for systemic venous return to passively flow into the pulmonary circulation in the absence of a ventricular pumping chamber. Although the strategy is not considered a repair or a ‘cure,’ it does allow for normalization of blood flow and creation of a system for sufficient oxygen delivery for survival. An alternative management strategy for HLHS is infant cardiac transplantation. A number of centres have excellent results with transplantation20; however, in the author’s view, the limited number of organs and the growing improvement in outcome for reconstruction makes the latter the more logical choice. Recently, an innovative technique has been described in which stenting of the ductus arteriosus in the cardiac catheterization laboratory and pulmonary artery banding has been performed, with a more traditional pulmonary arteryeaorta anastomosis and arch reconstruction performed at a later point in time in conjunction with a bidirectional Glenn. This has been labelled the ‘hybrid procedure’ in that therapy consists of both catheterdriven intervention and surgery.21 The theoretical benefits of this approach include elimination of the first-stage operation in infancy, with a potential reduction in early mortality. The initial experience with this approach is still developing and will have to demonstrate superior outcomes to the more traditional strategy of classic reconstruction in infancy before becoming widely accepted.22 The author’s preferred strategy for HLHS is the staged surgical reconstruction. Following stabilization with initiation of prostaglandin E1 infusion for maintenance of patency of the ductus arteriosus, the first stage, or Norwood operation, is undertaken. The goals of this procedure are to more permanently redirect pulmonary venous 560 Table 1 Reconstructive strategy for hypoplastic left heart syndrome Operation Age Objective Procedure Physiological impact Stage I (Norwood operation) Newborn Unimpaired mixing of systemic and pulmonary venous return Reliable, unobstructed flow from right ventricle into patent, newly constructed aorta Right ventricle as the systemic and pulmonic ventricle Right ventricle volume overload Peripheral oxygen saturation 75e85% Stage II (superior cavopulmonary connection) 4e6 months Incorporation of superior systemic venous return to the lungs as the source for oxygenation Stable, more reliable source of pulmonary blood flow Stage III (Fontan operation) 18 monthse3 years Incorporation of inferior systemic venous return into the lungs Atrial septectomy Proximal pulmonary artery to aorta anastomosis Reconstruction of the aortic arch arising from the right ventricle Creation of a reliable source for pulmonary blood flow (aorto-pulmonary shunt, or right ventricle to pulmonary artery conduit) Elimination of shunt or conduit Anastomosis of superior vena cava to the branch pulmonary arteries (bidirectional Glenn, or hemi-Fontan) Augmentation of branch pulmonary arteries as necessary Inferior vena cava flow channelled to the pulmonary arteries (Fontan operation, many modifications) Volume unloading of the right ventricle Peripheral oxygen saturation 80e85% Increased pulmonary blood flow Peripheral oxygen saturation O90% J. Rychik Hypoplastic left heart syndrome return to the right atrium and assign the right ventricle to the task of ejecting both systemic and pulmonic venous return into a reconstructed aorta. The ‘neo’-aorta is composed of the transected native proximal pulmonary artery which is anastomosed to the diminutive native ascending aorta. In order to fill out the full calibre of this ‘neo’-aorta, a gusset of pulmonary homograft is used to create an open and smooth transition in the vessel down to the descending aorta distal to the insertion site of the ductus arteriosus. An alternative approach is described in which natural aortic and pulmonic tissue alone is used to completely refashion the aorta.23 Once an open pathway has been created from the right ventricle to the descending aorta, a source for pulmonary blood flow is established. In the classic Norwood operation, a tube graft is inserted from the base of the innominate artery or directly from the reconstructed aorta to the branch right pulmonary artery (aorto-pulmonary shunt, modified BlalockeTaussig shunt). Recently, interest has been generated in the use of a small tube conduit from an incision in the body of the right ventricle to the central pulmonary arteries as a source of pulmonary blood flow (Sano modification).24 This modification may have some advantages in that, unlike the aorto-pulmonary shunt, there is no potential for competitive steal of blood flow away from the ascending aorta and coronary circulation. Preliminary data suggest an improved early survival for infants utilizing the Sano-modified approach.25 However, concerns about the long-term effects on right ventricular function of an incision in the body of the right ventricle should lead to caution in the widespread use of this approach. A randomized study sponsored by the National Institutes of Health looking at these two approaches is currently underway in the USA. The first stage of reconstruction is a technical challenge to the surgeon and a physiological challenge to the infant. Reconstruction of the aortic arch without proximal obstruction to coronary perfusion or distal obstruction to descending aortic flow is the goal. Physiologically, the right ventricle must carry the burden of support for both the systemic and pulmonic circulations. Experience in postoperative care by dedicated teams of physicians and nurses has dramatically improved outcomes during the fragile period immediately following surgery. Current survival outcomes for stage I are greater than 90% in many centres worldwide. Risk factors for poor outcome have been identified,3 and include prematurity, the presence of additional extracardiac anomalies or chromosomal anomalies, and the anatomical findings of an abnormal tricuspid valve with severe 561 regurgitation or an intact atrial septum. In the author’s experience, other structural factors such as the size of the ascending aorta or the subtype of anatomical HLHS have not influenced outcome. With improved stage I survival has come the identification of a growing interstage mortality, with approximately 5% out-of-hospital mortality after stage I but before stage II.26 The factors contributing to this mortality are unclear; however, its presence prompts the need to proceed with the stage II operation at the earliest possible point in time since mortality after stage II is limited. The stage II superior cavopulmonary anastomosis operation can be performed as soon as passive blood flow can be accommodated into the pulmonary vasculature. Pulmonary vascular resistance decreases progressively after birth, and reaches a point at which passive superior vena caval return can be directed into the pulmonary arteries at approximately 4e6 months of age. Two techniques currently exist: (1) the bidirectional Glenn, in which the superior vena cava is detached from the roof of the right atrium and connected end-toside to the right pulmonary artery; and (2) the hemi-Fontan, in which the superior vena cava is left in situ, a side-to-side anastomosis is created between it and the right pulmonary artery, and a patch of pulmonary homograft is used to create a dam to close the orifice of the superior vena cava into the right atrium. Outcomes for both techniques are excellent, with 99% survival in a number of series.27 The stage III operation, or Fontan operation, can be performed electively anywhere from 18 months to 3 years of age. A variety of modifications have evolved, with institutional-specific preferences dictating the style of operation. Common to all is the channelling of inferior vena caval flow into the pulmonary circulation. This can be achieved via an intra-atrial baffle or lateral tunnel technique, in which a patch of material is sewn around the orifice of the inferior vena cava with flow baffled towards the junction between the superior vena cava and the right pulmonary artery. Alternatively, a tube graft can be attached to the inferior vena cava at its entry into the right atrium, and then connected distally to the right pulmonary artery outside of the confines of the right atrium. This technique is known as the ‘extracardiac conduit’ type of Fontan operation. The potential benefit of this technique is the avoidance of atrial incision and atrial hypertension, which may reduce the incidence of late atrial arrhythmias commonly seen in the atrial lateral tunnel technique.28 An important modification to the Fontan operation is the creation of a fenestration or 562 J. Rychik communication between the systemic venous pathway and the pulmonary venous chamber. In the author’s experience, a 4e5-mm fenestration created at the time of the Fontan operation results in excellent postoperative outcome and a smooth postoperative course.29 The fenestration results in oxygen saturation that is slightly diminished due to right to left shunting, but ventricular filling and cardiac output are markedly enhanced; hence, such patients have improved oxygen delivery.30 While early peripheral oxygen saturations are in the mid-80% range, the fenestration commonly undergoes spontaneous closure over time. In the author’s experience, over three-quarters of fenestrations close spontaneously or are insignificantly small within 1 year of surgery.31 Thereafter, oxygen saturation levels are O90%. Survival following Fontan operation in the current era is excellent with 99% survival. School age: how are these children doing? The overall success of the strategy for reconstructive surgery for HLHS has resulted in a large number of children who are survivors; the majority are now at middle school. Most of the mortality following reconstruction of HLHS takes place soon after stage I or as an interstage death prior to the superior cavopulmonary connection. Late mortality after reconstruction is relatively uncommon. However, with an increasing number of children surviving the rigors of surgery, a number of morbidities have been identified (Table 2). Exercise limitation is a common finding in children with HLHS after Fontan operation. The ability to increase cardiac output in response to the demands of exercise is limited.32 The absence of a pulmonary pumping chamber to deliver a normal complement of blood to the lungs and the presence of a morphological right ventricle as the systemic ventricle contribute to an inability to appropriately increase stroke volume to match needs to the same degree seen in the normal two-ventricle heart. In Table 2 addition, the heart rate response to exercise is typically blunted after Fontan operation due to sinus node dysfunction. Many children with HLHS enjoy a number of sports activities, but they frequently tire easily and rarely enter high-level competition. Each child is encouraged to participate in sports and to seek out their own level of comfort, with no strict restrictions imposed. Atrial arrhythmias are common after Fontan operation and are likely to be related to the multiple incisions and suture lines present. Sinus node dysfunction with sinus bradycardia or junctional rhythm can be present as a consequence of the stage II or stage III operations, as these operations require manipulation near the sinus node with potential trauma to it or interruption of the arterial supply of the sinus node.33 Atrial flutter can be seen as a consequence of scarred atrial tissue and may sometimes be difficult to treat. Children after Fontan operation have a predilection towards thrombus formation.34 The cause of this phenomenon is multifactorial. A generalized low cardiac output state with stasis of blood and low flow velocity within the atria are contributing factors. Synthetic patch material is used for the lateral tunnel or conduit systemic venous pathway, which may act as a thrombogenic source. Atrial arrhythmias may predispose to clot formation. A number of investigators have documented abnormalities in coagulation proteins after Fontan operation.35 Recently, coagulation abnormalities have been discovered in infants with single-ventricle anatomy prior to Fontan operation, raising the possibility that these abnormalities are primary and associated, but not secondarily caused by the physiology of the Fontan circulation.36 Nonetheless, these children are at increased risk for stroke and pulmonary embolism. Recommendations for antiplatelet or anticoagulative therapies are controversial, with varying viewpoints on the correct management style and efficacy of these agents.37 Prophylactic treatment strategies range from daily aspirin (as an antiplatelet agent) to warfarin. Studies investigating the efficacy of these treatments are currently underway. Morbidities in children with hypoplastic left heart syndrome after Fontan operation Morbidity Frequency Exercise intolerance Arrhythmia Thrombo-embolic disease (e.g. pulmonary embolism, stroke) Protein-losing enteropathy Neurocognitive disabilities (e.g. learning differences, attention deficit/ hyperactivity disorder) Majority, to varying degrees 25e50%, to varying degrees Approximately 10% !5% 10e70%, to varying degrees Hypoplastic left heart syndrome Another enigmatic morbidity seen after Fontan operation is protein-losing enteropathy. Abnormal enteric protein loss can occur spontaneously, months or years after Fontan surgery, resulting in marked hypoproteinaemia. Clinically, these patients present with diarrhoea, abdominal discomfort, and peripheral oedema with ascites as a consequence of low serum protein levels.38 The disease occurs in 3e13% of cases, with nearly 50% mortality 5 years after diagnosis.39 The precise pathophysiological mechanism is unknown. Interventions that improve and maximize cardiac output, such as creation of a fenestration, have resulted in successful management of the disease, suggesting that the physiology of low cardiac output and high central venous pressure unique to the Fontan circulation may predispose to the ailment.40 However, questions remain regarding why certain patients suffer from protein-losing enteropathy and others with similar haemodynamics do not, raising the possibility of a predisposition to the disease in select patients, but only after the haemodynamics of the Fontan circulation are imposed. Of great interest has been the identification of neurocognitive difficulties in many of the children with HLHS now entering school age.41 A number of studies have demonstrated that these children face a variety of neurodevelopmental difficulties. In one series, mean scores on standardized psychometric tests for a group of HLHS children were significantly lower than the normal population, with 18% scoring less than 70 on intelligence quotient testing. Of note, nearly 70% met criteria for attention deficit/hyperactivity disorder based on neurological examination.42 In another study, children with HLHS after Fontan operation were compared with other children with a single ventricle after Fontan operation. Test scores for the HLHS group were found to be lower than those for the non-HLHS group, but were in the low-average range.43 Most interestingly, similar deficiencies have been identified in children with HLHS undergoing a strategy of heart transplantation,44 suggesting that these deficits may not necessarily be related to the style of treatment but are directly associated with HLHS. Children who undergo the reconstructive approach for HLHS are required to have three operations with consequential courses of deep hypothermic circulatory arrest; a variable that can impact negatively on neurocognitive outcome. However, emerging data indicate that the deficiencies seen in HLHS may be influenced by other factors. The duration of deep hypothermic circulatory arrest does not appear to correlate with the neurodevelopmental deficits found. Structural abnormalities of the brain have been identified in HLHS prior to surgery, including holoprosencephaly and agenesis of the 563 corpus callosum.45 A more subtle abnormality of brain dysgenesis noted is that of an underdeveloped operculum, the region of the cortical mantle at the juncture of the frontal, parietal and temporal lobes. Deficiencies in this region are associated with feeding and swallowing difficulties, and this is of interest as many infants exhibit problems with feeding after stage I surgery.46 Periventricular leukomalacia, a non-specific sign of cerebral white matter injury as seen on magnetic resonance imaging, is present in up to 16% of neonates with complex congenital heart disease prior to surgery, but in over 50% after surgery.47,48 This raises the possibility that neonates with HLHS have a fragile central nervous system, with inherent deficiencies present that may be further impacted upon by the potential neurological ‘injury’ of the procedures required for heart surgery. Recent data on the response to brain injury after cardiopulmonary bypass demonstrate a relationship with a gene polymorphism of the apo-lipoprotein E, a molecule that plays an important role in neuronal repair. Following heart surgery, infants with apo-lipoprotein E epsilon 2 allele are at greater risk of having significantly lower psychomotor development indices at 1 year of age than infants with the other genotypes for this protein.49 These data further support the notion of certain patients being at risk for neurodevelopmental dysfunction with a predilection towards poor response to nervous system injury, such as cardiopulmonary bypass. Whether infants with HLHS are at such an increased risk is speculative at this point. Linking the data on fetal cerebrovascular flow patterns and cerebrovascular resistance in the fetus with HLHS with the subtle structural findings and genetic predisposition to injury that exists may ultimately result in stratification of the infant at most significant risk for neurological injury. Specific changes in neuroprotective strategy may then be implemented for these high-risk patients. Putting it all into perspective: counselling a family carrying a fetus with HLHS Considering the outcome data on morbidity and mortality as we know it today, how should one counsel the family of a fetus with HLHS? Geographic, cultural and religious factors influence the response when a family is faced with the news of carrying a fetus with HLHS. Unfortunately, because of the history related to poor outcome in the past, many practitioners are unaware of the current survival statistics for HLHS. National or regional experiences may also impact either 564 positively or negatively on the statistics and data transmitted to the family. Not all centres are uniformly able to offer the same survival statistics based on their own experiences. This raises the question of regionalization of care and referral to centres of excellence specifically identified as able to treat this anomaly. The facts support the notion that survival is possible for the majority when the procedure and postoperative care are performed by experienced hands. Lack of knowledge by primary obstetricians and maternalefetal medicine experts concerning these recent statistics can result in inaccurate transfer of information to families. While excellent operative statistics can now be quoted, counselling sessions should focus more extensively on the issues of long-term morbidity and the risks of unknown beyond the second decade of life, for which few data are available. In the author’s view, counselling must be offered by knowledgeable physicians/nurses in a non-biased factual manner, providing as much objective information as possible. Family support should be offered regardless of the decisions made. Development of a multidisciplinary approach to counselling these families is helpful. The author’s group has constructed a Fetal Heart Program that includes cardiologists, nurses, cardiac surgeons and maternalefetal medicine specialists, all of whom contribute to the evaluation and assistance of these families as they make their way forward in gestation. Bringing potential parents into contact with other families those have gone through the decision process can be helpful. For those families who choose to continue the pregnancy, delivery can be safely performed vaginally with prostaglandin initiated after birth. The author’s group routinely perform serial fetal echocardiographic studies for fetuses with HLHS at 4-week intervals after initial prenatal diagnosis to survey for any changes in tricuspid valve regurgitation, or restriction in the atrial septum. These serial sessions also offer the opportunity for further educational and psychological support for the parents and other family members. A dedicated nurse co-ordinator in our programme assists with shepherding the families through the prenatal process, creating a smooth transition to the environment of the intensive care unit. Recent data suggest that such a process can alleviate some of the stress related to carrying a fetus with HLHS.50 Prenatal diagnosis of HLHS has also been shown to result in improved physiological state prior to surgery51 and improved surgical outcome.52 The potential impact of prenatal diagnosis of HLHS on some of the long-term morbidities listed above, particularly neurocognitve outcome, is unknown; however, a positive influence is expected. J. Rychik Practice points 1) Currently multiple techniques for palliation of HLHS with relatively good survival 2) Neurocognitive testing and surveillance is indicated in children with HLHS Research directions 1) Improved understanding of the development of HLHS during fetal life 2) Long-term follow up and adult outcome of survivors of HLHS References 1. Spray TL. Stage I reconstruction. In: Rychik J, Wernovsky G, editors. Hypoplastic left heart syndrome. Boston/ Dordrecht/London: Kluwer Academic Publishers; 2003. p. 89e105. 2. Mahle WT, Spray TL, Wernovsky G, Gaynor JW, Clark 3rd BJ. Survival after reconstructive surgery for hypoplastic left heart syndrome: a 15-year experience from a single institution. Circulation 2000;102(Suppl. 3):III136e41. 3. Gaynor JW, Mahle WT, Cohen MI, Ittenbach RF, DeCampli WM, Steven JM, et al. Risk factors for mortality after the Norwood procedure. Eur J Cardiothorac Surg 2002;22:82e9. 4. Hoffman JI, Kaplan S, Liberthson RR. Prevalence of congenital heart disease. Am Heart J 2004;147:425e39. 5. Natowicz M, Kelley RI. Association of Turner’s syndrome with hypoplastic left heart syndrome. Am J Dis Child 1987;141:218e20. 6. Natowicz M, Chatten J, Clancy R, Conard K, Glauser T, Huff D, et al. Genetic disorders and major extra-cardiac anomalies associated with the hypoplastic left heart syndrome. Pediatrics 1988;82:698e706. 7. Boughman JA, Berg KA, Astemborski JA, Clark EB, McCarter RJ, Rubin JD, et al. Familial risks of congenital heart defects assessed in a population based epidemiologic study. Am J Med Genet 1987;26:839e49. 8. Rosenthal GL. Patterns of prenatal growth among infants with cardiovascular malformations: possible fetal hemodynamic effects. Am J Epidemiol 1996;143:505e13. 9. Hornberger LK, Sahn DJ, Kleinman CS, Copel JA, Reed KL. Tricuspid valve disease with significant tricuspid insufficiency in the fetus: diagnosis and outcome. J Am Coll Cardiol 1991; 17:167e73. 10. Levin MD, Gaynor JW, Tian Z, Cohen MS, Donaghue DD, Spray TL, et al. Prevalence of perinatal atrioventricular valve regurgitation in the single ventricle: from the fetus, through birth, and initial palliative surgery. J Am Soc Echocardiogr 2004;17:495 [abstract]. 11. Rasanen J, Wood DC, Weiner S, Ludomirski A, Huhta JC. Role of the pulmonary circulation in the distribution of human fetal cardiac output during the second half of pregnancy. Circulation 1996;94:1068e73. Hypoplastic left heart syndrome 12. Rychik J, Rome JJ, Collins MH, DeCampli WM, Spray TL. The hypoplastic left heart syndrome with intact atrial septum: atrial morphology, pulmonary vascular histopathology and outcome. J Am Coll Cardiol 1999;34:554e60. 13. Chintala K, Tian Z, Donaghue DD, Thomas RL, Rychik J. Fetal pulmonary venous Doppler patterns in hypoplastic left heart syndrome: relationship to atrial septal restriction. J Am Coll Cardiol 2004;43(Suppl. A):381A [abstract]. 14. Vlahos AP, Lock JE, McElhinney DB, van der Velde ME. Hypoplastic left heart syndrome with intact or highly restrictive atrial septum: outcome after neonatal transcatheter atrial septostomy. Circulation 2004;109:2326e30. 15. Marshall AC, van der Velde ME, Tworetzky W, Gomez CA, Wilkins-Haug L, Benson CB, et al. Creation of an atrial septal defect in utero for fetuses with hypoplastic left heart syndrome and intact or highly restrictive atrial septum. Circulation 2004;110:253e8. 16. Donofrio MT, Bremer YA, Schieken RM, Gennings C, Morton LD, Eidem BW, et al. Autoregulation of cerebral blood flow in fetuses with congenital heart disease: the brain sparing effect. Pediatr Cardiol 2003;24:436e43. 17. Kaltman JR, Di H, Tian Z, Rychik J. Impact of congenital heart disease on cerebrovascular blood flow dynamics in the fetus. Ultrasound Obstet Gynecol 2005;25:32e6. 18. Shillingford AJ, Marino BS, Ittenbach RF, Fedec A, Rychik J, Clancy RR, et al. Microcephaly is common in neonates with hypoplastic left heart syndrome. Pediatr Cardiol 2005;25:581A. 19. Norwood WI, Lang P, Hansen DD. Physiologic repair of aortic atresia e hypoplastic left heart syndrome. N Engl J Med 1983;308:23e6. 20. Chrisant MR, Naftel DC, Drummond-Webb J, Chinnock R, Canter CE, Boucek MM, et al. Fate of infants with hypoplastic left heart syndrome listed for cardiac transplantation: a multicenter study. J Heart Lung Transplant 2005;24:576e82. 21. Akintuerk H, Michel-Behnke I, Valeske K, Mueller M, Thul J, Bauer J, et al. Stenting of the arterial duct and banding of the pulmonary arteries: basis for combined Norwood stage I and II repair in hypoplastic left heart. Circulation 2002;105: 1099e103. 22. Galantowicz M, Cheatham JP. Lessons learned from the development of a new hybrid strategy for the management of hypoplastic left heart syndrome. Pediatr Cardiol 2005;26: 190e9. 23. Ishino K, Stumper O, De Giovanni JJ, Silove ED, Wright JG, Sethia B, et al. The modified Norwood procedure for hypoplastic left heart syndrome: early to intermediate results of 120 patients with particular reference to aortic arch repair. J Thorac Cardiovasc Surg 1999;117:920e30. 24. Sano S, Ishino K, Kado H, Shiokawa Y, Sakamoto K, Yokota M, et al. Outcome of right ventricle-to-pulmonary artery shunt in first-stage palliation of hypoplastic left heart syndrome: a multi-institutional study. Ann Thorac Surg 2004;78:1951e7. 25. Pizarro C, Mroczek T, Malec E, Norwood WI. Right ventricle to pulmonary artery conduit reduces interim mortality after stage 1 Norwood for hypoplastic left heart syndrome. Ann Thorac Surg 2004;78:1959e63. 26. Mahle WT, Spray TL, Gaynor JW, Clark 3rd BJ. Unexpected death after reconstructive surgery for hypoplastic left heart syndrome. Ann Thorac Surg 2001;71:61e5. 27. Karl TR. The bidirectional cavopulmonary shunt. In: Rychik J, Wernovsky G, editors. Hypoplastic left heart syndrome. Boston/Dordrecht/London: Kluwer Academic Publishers; 2003. p. 129e49. 28. Nurnberg JH, Ovroutski S, Alexi-Meskishvili V, Ewert P, Hetzer R, Lange PE. New onset arrhythmias after the extracardiac conduit Fontan operation compared with the 565 29. 30. 31. 32. 33. 34. 35. 36. 37. 38. 39. 40. 41. 42. 43. 44. 45. intraatrial lateral tunnel procedure: early and midterm results. Ann Thorac Surg 2004;78:1979e88. Gaynor JW, Bridges ND, Cohen MI, Mahle WT, DeCampli WM, Steven JM, et al. Predictors of outcome after the Fontan operation: is hypoplastic left heart syndrome still a risk factor? J Thorac Cardiovasc Surg 2002;123:237e45. Hijazi ZM, Fahey JT, Kleinman CS, Kopf GS, Hellenbrand WE. Hemodynamic evaluation before and after closure of fenestrated Fontan. An acute study of changes in oxygen delivery. Circulation 1992;86:196e202. Yang SG, Clark BJ, Gaynor JW, Spray TL, Rychik J. Rate of spontaneous closure of fenestration in the modified Fontan operation. Circulation 1999;100(Suppl. 1):I-399. Joshi VM, Carey A, Simpson P, Paridon SM. Exercise performance following repair of hypoplastic left heart syndrome: a comparison with other types of Fontan patients. Pediatr Cardiol 1997;18:357e60. Cohen MI, Rhodes LA. Sinus node dysfunction and atrial tachycardia after the Fontan procedure: the scope of the problem. Semin Thorac Cardiovasc Surg Pediatr Card Surg Annu 1998;1:41e52. Coon PD, Rychik J, Novello RT, Ro PS, Gaynor JW, Spray TL. Thrombus formation after the Fontan operation. Ann Thorac Surg 2001;71:1990e4. Odegard KC, McGowan Jr FX, Zurakowski D, Dinardo JA, Castro RA, del Nido PJ, et al. Procoagulant and anticoagulant factor abnormalities following the Fontan procedure: increased factor VIII may predispose to thrombosis. J Thorac Cardiovasc Surg 2003;125:1260e7. Odegard KC, McGowan Jr FX, Zurakowski D, DiNardo JA, Castro RA, del Nido PJ, et al. Coagulation factor abnormalities in patients with single-ventricle physiology immediately prior to the Fontan procedure. Ann Thorac Surg 2002;73:1770e7. Jacobs ML, Pourmoghadam KK, Geary EM, Reyes AT, Madan N, McGrath LB, et al. Fontan’s operation: is aspirin enough? Is coumadin too much? Ann Thorac Surg 2002;73: 64e8. Rychik J, Spray TL. Strategies to treat protein-losing enteropathy. Semin Thorac Cardiovasc Surg Pediatr Card Surg Annu 2002;5:3e11. Mertens L, Hagler DJ, Sauer U, Somerville J, Gewillig M. Protein-losing enteropathy after the Fontan operation: an international multicenter study. J Thorac Cardiovasc Surg 1998;115:1063e73. Rychik J, Rome JJ, Jacobs ML. Late surgical fenestration for complications after the Fontan operation. Circulation 1997; 96:33e6. Wernovsky G, Shillingford AJ, Gaynor JW. Central nervous system outcomes in children with complex congenital heart disease. Curr Opin Cardiol 2005;20:94e9. Mahle WT, Clancy RR, Moss EM, Gerdes M, Jobes DR, Wernovsky G. Neurodevelopmental outcome and lifestyle assessment in school-aged and adolescent children with hypoplastic left heart syndrome. Pediatrics 2000;105:1082e9. Goldberg CS, Schwartz EM, Brunberg JA, Mosca RS, Bove EL, Schork MA, et al. Neurodevelopmental outcome of patients after the fontan operation: a comparison between children with hypoplastic left heart syndrome and other functional single ventricle lesions. J Pediatr 2000;137:646e52. Ikle L, Hale K, Fashaw L, Boucek M, Rosenberg AA. Developmental outcome of patients with hypoplastic left heart syndrome treated with heart transplantation. J Pediatr 2003;142:20e5. Glauser TA, Rorke LB, Weinberg PM, Clancy RR. Congenital brain anomalies associated with the hypoplastic left heart syndrome. Pediatrics 1990;85:984e90. 566 46. Clancy R. Neurological issues. In: Rychik J, Wernovsky G, editors. Hypoplastic left heart syndrome. Boston/Dordrecht/ London: Kluwer Academic Publishers; 2003. p. 251e73. 47. Mahle WT, Tavani F, Zimmerman RA, Nicolson SC, Galli KK, Gaynor JW, et al. An MRI study of neurological injury before and after congenital heart surgery. Circulation 2002; 106(Suppl. 1):I109e14. 48. Galli KK, Zimmerman RA, Jarvik GP, Wernovsky G, Kuypers MK, Clancy RR, et al. Periventricular leukomalacia is common after neonatal cardiac surgery. J Thorac Cardiovasc Surg 2004;127:692e704. 49. Gaynor JW, Gerdes M, Zackai EH, Bernbaum J, Wernovsky G, Clancy RR, et al. Apolipoprotein E genotype J. Rychik and neurodevelopmental sequelae of infant cardiac surgery. J Thorac Cardiovasc Surg 2003;126:1736e45. 50. Sklansky M, Tang A, Levy D, Grossfeld P, Kashani I, Shaughnessy R, et al. Maternal psychological impact of fetal echocardiography. J Am Soc Echocardiogr 2002;15:159e66. 51. Verheijen PM, Lisowski LA, Stoutenbeek P, Hitchcock JF, Brenner JI, Copel JA, et al. Prenatal diagnosis of congenital heart disease affects preoperative acidosis in the newborn patient. J Thorac Cardiovasc Surg 2001;121:798e803. 52. Tworetzky W, McElhinney DB, Reddy VM, Brook MM, Hanley FL, Silverman NH. Improved surgical outcome after fetal diagnosis of hypoplastic left heart syndrome. Circulation 2001;103:1269e73.