* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download electron-microscope observations on cell nuclei in various tissues of
Survey
Document related concepts
Transcript
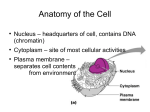
J. Cell Sci. ai, 315-327 (1976) Printed in Great Britain 315 ELECTRON-MICROSCOPE OBSERVATIONS ON CELL NUCLEI IN VARIOUS TISSUES OF A TELEOST FISH: THE NUCLEOLUS-ASSOCIATED MONOLAYER OF CHROMATIN STRUCTURAL UNITS H. G. DAVIES AND M. E. HAYNES MRC Cell Biophysics Unit, 26-29 Drury Lane, London, W.C. 2, England SUMMARY Observations on stain uptake by thin sections through condensed interphase chromosomes in cells from epithelial and muscle tissue in kidney and intestine, and also infibroblasts,show a distribution into DNA-rich and DNA-poor phases similar to that already described in cells from the connective tissue blood. In all the nuclei the nucleolus, when adjacent to the nuclear envelope, is separated from the inner membrane by a monolayer of chromatin structural units, similar to the monolayer enclosed on both sides by nuclear envelope, previously described in a wide variety of organisms. The data provide further support for the hypothesis that the condensed interphase chromosomes in eukaryotes are characterized by essentially similar structural units folded to form similar patterns. This hypothesis, regarding the higher order units, is consistent with data of others which show that histones and DNA fold to form similar repeating subunits in chromatin, irrespective of the base sequence in the DNA and the origin of the histones. INTRODUCTION Our previous electron-microscope observations on the structure of condensed interphase chromosomes in situ, the chromatin bodies, have been largely confined to various types of blood cell, in particular chicken erythrocytes and reticulocytes (references in Davies, Murray & Walmsley, 1974). To explain the staining patterns seen (Fig. 1) when thin sections are treated with heavy molecules which bind preferentially either to DNA or protein, it has been proposed that chromatin consists of 2 different regions or phases, the one DNA-rich, the other DNA-poor and most probably with a higher ratio of protein to DNA. To account for these patterns and other data (Walmsley & Davies, 1975), it was suggested that condensed chromatin consists of thread-like nucleoprotein units, total diameter about 28-0 nm with an inner DNArich core about 17-0 nm in diameter. Contact with the nuclear envelope has an orienting effect on the units, resulting in side-by-side packing to form one or more layers. The purpose of this paper is to show that there is a similar organization in the condensed interphase chromosomes of cells from 2 tissues of a teleost fish, other than blood, namely epithelial and muscle, as well as in a second kind of connective tissue cell. Attention is also drawn to another general feature of nuclear morphology, found in all 316 H. G. Davies and M. E. Haynes the cell types so far examined; when the nucleolus lies adjacent to the envelope it is invariably separated from it by a sheet of chromatin, similar to the monolayers confined on both sides by nuclear envelope, previously described in animal and plant cells (references in Davies & Haynes, 1975). Fig. 1. A, schematic diagram showing ultxaetructure of a thin section through a nucleus. In the chromatin bodies, after uranyl-lead there are dense dots and dashes (DNA-rich) separated by regions which stain less (DNA-poor). The thread-like nucleoprotein units pack side-by-side at the surface of the nucleus into one or more layers shown in B. rm and om, inner and outer membranes of the nuclear envelope. B, b2, b4 are the bands containing the dots and dashes; bi,bs,b^are the bands with lower stain uptake. Hence the DNA-rich regions are referred to as the e-phase (evenly numbered) and the DNA-poor regions as the o-phase. d is the width of the uranyl-lead dense regions and a is the separation of the units seen end-on. Data on 17-day-embryo chicken erythrocytes (Davies & Small, 1968) show d averages 17-4 nm (range ± 3 nm), a averages 280 ran (range ± 4 nm). c, a monolayer of unit threads (previously referred to as superunit threads in Davies et al. 1974) separating the nucleolus from the inner membrane. The subscript n refers parameters in B to the nucleolus; ng and nf, its granular and fibrillar areas respectively. MATERIALS AND METHODS Specimens of goldfish, Carassius auratus, were stunned and pieces of kidney and intestine were processed by conventional procedures. Fixative 1 was 3 % glutaraldehyde in o-1 M cacodylate buffer, pH 72, with 0-002 M CaCl,, followed by 1 % OsO4 in a similar medium. Fixative 2 was 1-5 % acrolein, 1 % potassium dichromate and 6 % sucrose, pH adjusted to 75 with 5N KOH followed by Millonig's (1961) 1 % phosphate-buffered OsO4 according to the procedures in Robison & Lipton (1969). Sections of Araldite-embedded material were cut with diamond knives on an A. F. Huxley-Cambridge ultramicrotome. Staining was in 2 % magnesium uranyl acetate for 20 min at 60° C, followed by lead citrate (Reynolds, 1963) for 5 min; or in 2 % phosphotungstic acid (PTA) in 90% ethanol, 10% water, for 30 min at Organization of interphase nuclei 317 room temperature; or in 0 9 % potassium permanganate ino-iM phosphate buffer, pH 6-5, for 30 min (Soloff, 1973; Davies, 1976). Sections were examined in a Siemens Elmiskop 1 operated at 100 kV with a so-/tm objective aperture. RESULTS The cells in vertebrates are organized into 4 basic tissues (Bloom & Fawcett, 1968): epithelial, muscle, connective and nervous. We examined cells from the first 3 types contained in 2 organs, intestine and kidney. The intestine of the goldfish, like that of the rainbow trout (Weinreb & Bilstad, 1955), is composed of 3 major layers, the mucosa the encircling muscularis and the external serosa. The mucosa is arranged in folds, the surface epithelial cells confining a loose connective tissue, the tunica propria, which contains numerous blood vessels of varying size and hence complexity of organization. The muscularis consists predominantly of smooth muscle cells, interspersed by occasional connective tissue cells, namely fibroblasts, adjacent to which are collagen fibres. The kidney of goldfish consists of well defined tubules lined with epithelial cells and between them lie haematopoietic tissues consisting of immature granulocytes and erythrocytes; blood vessels are also located in these areas. A description of the dot—dash pattern, seen in the chromatin bodies of 17-day embryonic erythrocytes after staining with uranyl-lead is given in Fig. 1, p. 316. The geometry of the pattern was similar in all the nuclei of cells from goldfish after either fixation 1 or 2 (Methods). Epithelial tissue: endothelial cells References to the organization of blood vessels in the mammalian vasculature are given by Bruns & Palade (1968) in their paper on blood vessels in skeletal muscle. In general, the walls consist of 3 concentric layers and the small vessels examined in goldfish had a similar structure. The inner tunic consists of a single layer of flattened endothelial cells whose inner surface is in contact with the blood and whose outer surface is covered by a basement membrane (Fig. 3). Apart from their location, endothelial cells are also characterized by small vesicles in the cytoplasm near the plasma membrane. As well as the usual organelles the cytoplasm also contains particles of glycogen (Fig. 2) and infrequent bundles of fibrils, diameter roughly 5-0 nm. The nucleus is elongated, contains deep clefts (Fig. 3) and much condensed chromatin. Bands bi, b2, and 63 are visible around the entire periphery of the nucleus wherever the nuclear envelope is sectioned normally. Additional bands up to by can sometimes be found (Fig. 2). When a thin section is treated with ethanolic PTA (Fig. 4) the stain distribution in the condensed chromatin is approximately uniform, apart from a denser zone of stain adjacent to the nuclear envelope and around each body, as in the /?-pattern previously described (fig. 6 in Davies et al. 1974). After staining with KMnO4 the dot-dash pattern was also difficult to distinguish and the denser-staining zone was absent (Davies, 1976). Both KMnO4 and PTA preferentially bind to protein, whereas uranyl-lead preferentially binds to DNA; the patterns are discussed in Davies et al. (1974). The nucleolus of the endothelial cell is small and has 2 components, one granular, H. G. Davies and M. E. Haynes • • * * : Organization of interphase nuclei 319 the other finely fibrillar. In these nucleoli and in those from other tissues small areas of condensed chromatin lie within the fibrillar region or at its boundary with the granular area (Fig. 2). Invariably when the nucleolus lies adjacent to the nuclear envelope it is separated by bands bin, b2w bjn (Figs 1, 2) similar in geometry to the monolayers of unit threads enclosed on both sides by nuclear envelope previously described in the blood cells of goldfish and elsewhere (Davies & Haynes, 1975). Band bjn is not always clearly seen, due presumably to it taking up stain to roughly the same extent as the nucleolus. Band b2n may be continuous or broken up into dots, separation about 28-0 nm (see especially Fig. 5). There is no preferential location of either the granular or fibrillar zones with respect to the nucleolus-associated monolayer in either the endothelial cells or in any of the other cell types described below. Epithelial tissue in kidney and intestine The epithelial cells lining kidney tubules are joined together by cell junctions (Fig. 5). As in endothelial cells, condensed chromatin covers the interior surface of the nuclear envelope except at the pores, but it occupies a smaller fraction of the nuclear volume and the nucleoli are larger, both of which facts are consistent with greater activity of the nucleus in the epithelial cell. The nucleolus-associated monolayer is present. The nuclei of the epithelial cells lining the intestine (Fig. 8) have a similar structure. Muscle tissue The middle tunic of the capillaries consists of the so-called basement membrane and includes the pericytes (Fig. 3) by virtue of their location entirely within it (Bruns & Palade, 1968). In larger vessels (Rhodin, 1967) smooth muscle cells occupy this position and such a cell with collagenous components of the basement membrane is shown in Fig. 7. The muscle cell is characterized by an abundance of fine filaments in the cytoplasm, and glycogen particles are also common. Their nuclei, like those of the endothelial cell, are elongated and the dot-dash pattern in the condensed chromatin, seen clearly after binding uranyl-lead, is difficult to distinguish after either PTA or KMnO4. The nucleoli are mainly finely fibrillar and separated from the nuclear envelope by the usual monolayer. In the muscularis of goldfish intestine the smooth muscle cells have a structure All micrographs except Fig. 6 are from goldfish. All sections except Fig. 4 were stained with uranyl-lead. The nucleolus-aasociated layers are opposite the doubleheaded arrows. All cells were fixed in acrolein-dichromate followed by OsO 4 , except Figs. 6, 8, 9 which were fixed in glutaraldehyde-OsO 4 . Fig. 2. Electron micrograph showing part of the endothelial cell shown at low power in Fig. 3. nf, ng, fibrillar and granular components of the nucleolus; electron-dense bands opposite numbered arrows; /, lumen of capillary; condensed chromatin in nucleolus at arrow, x 120000. Fig. 3. Electron micrograph of an endothelial cell lining a blood capillary in the tunica propria of intestine, i, intestinal epithelial cell; I, lumen; p, pericyte; basement membrane at arrows, x 24000. 320 H. G. Dairies and M. E. Havnes Fig. 4. Electron micrograph of similar cell to Fig. 3 but stained with PTA. The chromatin bodies stain less and are more homogeneous than the nuclear sap; the denser zone is arrowed; cytoplasmic vesicles opposite lines; /, lumen of vessel, x 54000. Fig. 5. Electron micrograph of an epithelial cell from kidney tubule with cell junctions at arrows, x 23 500. Organization of interphase nuclei 321 \ '• \ Fig. 6. Electron micrograph of a reticulocyte from peripheral blood of 4-day chick. Ribosomes at arrows; n, nucleolus. x 100000. Fig. 7. Electron micrograph of smooth muscle cell from the wall of a vessel in the tunica propria of intestine, c, collagen fibrils; g, glycogen; n, nucleolus. x 87000. 3 22 H. G. Davies and M. E. Haynes .. . • Organization of interphase nuclei 323 similar to that just described, except that the cytoplasm contains dense bodies (Fig. 9) which may have a cohesive function (Bloom & Fawcett, 1968). In the nuclei up to 3 layers of units (bi-bj) have been counted. For reasons which are not clear, such multilayers are especially obvious at the ends of elongated nuclei, or where the radius of curvature is small (see also Fig. 2). Connective tissue: fibroblasts The dot- dash pattern is especially clear in the uranyl-lead treated fibroblast from the intestinal muscularis (Fig. 9). When the nuclear envelope is sectioned tangentially, longer segments of the units are seen (see also Everid, Small & Davies, 1970). Nucleolus-associated monolayers were also present (unpublished results) in the different kinds of granulocytes previously described in goldfish (Davies & Haynes, 1975), and are also found in blood cells of birds (Fig. 6). DISCUSSION Structural units in situ and in spread preparations: dimensions Evidence that DNA is folded up in association with histones to form structural units with basically similar dimensions and a common mode of packing in condensed interphase chromosomes of eukaryotes comes from several sources. First there are the sheets of chromatin limited by nuclear envelope commonly associated with nuclei of irregular shape (Haynes & Davies, 1973), to be found in a wide variety of organisms (references in Davies & Haynes, 1975): these sheets are of approximately constant width irrespective of species, plant or animal, consistent with their being a monolayer of similar units. Secondly, the data in this paper show that the staining patterns in the chromatin bodies, especially the mono- and multilayers adjacent to the envelope, from muscle and epithelium are essentially similar to those described in bird reticulocytes and other blood cells. This systematic study extends and places on a firm basis the earlier observation (Davies, 1968) of a few bands seen occasionally in published micrographs of cells other than blood. And further, there are the nucleolus-associated monolayers described here. The actual boundaries of individual unit threads cannot be distinguished in intact well fixed chromatin bodies due to close packing and interaction between them, but their outside diameter can be deduced from their centre-to-centre spacing in any one layer. The value, about 28-0 nm agrees with that deduced from the spacing between the inner membranes confining the monolayers (Davies et al. 1974). The value is also similar to that found for the fibres formed when interphase nuclei and mitotic chromo- Fig. 8. Electron micrograph of epithelial cell from intestine. Areas within the nucleolus staining like chromatin are arrowed, nf, ng,fibrillarand granular areas of nucleolus. x 54000. Fig. 9. Electron micrograph showing part of a fibroblast in the muscularis of intestine. c, collagen fibrils; d, dense region in the cytoplasm of a smooth muscle cell; surface view of nucleus at arrow, x 26000. 324 H. G. Davies and M. E. Haynes somes are spread on an air-water interface, namely 20-30 nra (references in Solari, 1974), which shows that separation of units is not accompanied by large changes in dimensions. However, in a high-voltage electron-microscope study on the spread fibres, Ris (1975) found no evidence for the differential packing or molecular composition described in situ (Davies et al. 1974), suggesting possible conformational changes on disruption. Previous measurements of widths of fibres, or threads, in thin sections through chromatin bodies summarized by Solari (1974) show most in the range from 8-o to 20-0 nm. Ris & Kubai (1970) showed clearly that thick fibres (~ 20-0 nm) give rise to thin fibres (~ io-o nm) when chromosomes are spread on solutions containing chelating agents. They proposed that the chelating action of buffers could give rise to the thin fibres reported in sections, but this was not the experience of Zirkin & Wolfe (1972). Zirkin & Sun-Kee Kim (1972) suggested that no one diameter can be considered to characterize the chromatin of eukaryotic nuclei, a view at variance with the data here derived from layers. Since edges of fibres are ill-defined their diameter must be more difficult to estimate accurately than that of spacings. Several new lines of evidence (Olins & Olins, 1973; Woodcock, 1973; Hewish & Burgoyne, 1973; Burgoyne, Hewish & Mobbs, 1974; Korngerg & Thomas, 1974; Kornberg, 1974; Van Holde, Sahasrabuddhe & Shaw, 1974; Noll, 1974; Baldwin, Bosely & Bradbury, 1975; Olins, Carlson & Olins, 1975; Oudet, Gross-Bellard & Chambon, 1975) have suggested a beads-on-a-string model for chromatin. From electron microscopy, the diameter of the beads or nucleoprotein particles, called ^-bodies (Olins & Olins, 1973) or nucleosomes (Oudet et al. 1975), ranges from about 6-o to 13-0 nm, which variation is quite likely due to well-known problems in size determination rather than an intrinsic difference in dimension. Clearly, therefore, the structural units in situ must be a higher-order structure formed by folding the beadson-a-string. In favourable orientations of the thin section the DNA-rich regions are annular in end-on view, split into two in side-on view, the so-called microtubes (Davies, 1968). This, and their thread-like nature, is consistent with some type of helical arrangement of the repeating unit. The repeating unit consists of about 200 base pairs of DNA with 2 each of the histones f2a1, f2a2, f2b and f3 (Kornberg, 1974; Thomas & Kornberg, 1975) and it has been suggested that the DNA is wound on the outside of a protein core (Kornberg, 1974; Van Holde et al. 1974). Part of this DNA, about 60 base pairs, may be loosely bound, since there often appear strands interconnecting the particles, and oligomers of particles exhibit a biphasic melting curve, the lower temperature corresponding to that for pure DNA (Woodcock, Frado & Sweetman, 1975). Further, the particles containing about 200 base pairs can be further digested to a 140 base-pair fragment (Sollner-Webb & Felsenfeld, 1975). The structural unit deduced from thin sections has a DNA-rich interior. If it is indeed formed from v-bodies, or nucleosomes, without much conformational change, then these bodies may have a DNA-rich inward-pointing face, perhaps containing this more loosely bound DNA. There is evidence that the repeating units can be reconstructed from chromatin proteins and DNA, irrespective of the origin of the DNA (viral, bacterial or homolo- Organization of interphase nuclei 325 gous) or the origin of the histones (Axel, Melchior, Sollner-Webb & Felsenfeld, 1974; Oudet et al. 1975). Evidently, assembly of the v-bodies, or nucleosomes, depends on histone interactions and does not involve recognition by histones of specific DNA base sequences. These results are consistent with our findings that the higher order units found in situ have dimensions and properties of further folding which are independent of cell type in eukaryotes. The v-bodies have not been distinguished in embedded material, perhaps due to close packing and low contrast. Nucleolus-associated monolayers The nucleolus (Smetana & Busch, 1974; Bouteille, Laval & Dupuy-Coin, 1974) is known to be a processing site for the ribosomal RNA transcribed from the r-DNA of the nucleolar-organizing chromosome. Most likely, therefore, the monolayer of units separating the nucleolus from the inner membrane of the envelope is part of this chromosome. We envisage that an increase in nucleolar products results in the displacement of unit threads away from the envelope. A similar event is the insertion of nuclear membranes between the 62 and bj layers resulting in the formation of the c--n--n monolayer (Haynes & Davies, 1973). The significance of the continued attachment of layer b2 to the envelope is not clear, but in general in eukaryotic nuclei there is complete coverage of the inner membrane except at the pores; areas free of chromatin become visible only during prophase (see, for example, fig. 15 in Davies & Tooze, 1966). A nucleolus-associated layer has previously been reported in 2 cells, in a newt polychromatic erythroblast (Davies, 1968) and in a human lymphoblast (Tokuyasu, Madden & Zeldis, 1968). The present study suggests the layer to be a characteristic feature of the eukaryotic nucleus. The nucleolus in general has a granular component and a fibrillar component which latter, in some somatic cells, can be resolved into dark fibrillar areas and light fibrillar areas or centres. Three zones are seen clearly after molecular segregation following certain treatments (Je'ze'quel, Shreeve & Steiner, 1967). RNA labelling experiments, for example on unfertilized eggs of Urec/iis caupo (Das, Micou-Eastwood, Ramamurthy & Alfert, 1970), showed that the production of ribosomal RNA precursor (38 s) and its first cleavage occur at the fibrillar core of the nucleous. The products, predominantly 30 S RNA, are then transported and stored in the granular cortex. A similar precursor-product relationship was found by Macgregor (1967) in nucleoli from Triturus oocytes: there the fibrillar component is preferentially located on that side of the nucleolus which lies nearest to the nuclear envelope. The nucleolus is separated from the envelope in small oocytes by a Feulgen-positive granule, the source - it is suggested (Macgregor, 1967) - of the r-DNA which spins out from it and lies within the fibrillar core. In the somatic cells described in this paper there is no preferential location of either the fibrillar zone or its granular products with respect to the envelope. The unit threads packed side-by-side are typical of those in inactive condensed chromatin, which is consistent with the suggestion that the monolayer is an attachment site, the r-DNA itself being located in the fibrillar zone of the nucleolus. 326 H. G. Davies and M. E. Haynes We thank Professor M. H. F. Wilkins, F.R.S., for encouragement, Mrs Yvonne Buchner for skilled assistance in specimen preparation and Mr Z. Gabor for help in preparing the plates. REFERENCES AXEL, R., MELCHIOR, W., SOLLNER-WEBB, B. & FELSENFELD, G. (1974). Specific sites of inter- action between histones and DNA in chromatin. Proc. natn. Acad. Sci. U.S.A. 71, 4101BALDWIN, J. P., BOSELEY, P. G. & BRADBURY, E. M. (1975). The subunit structure of the eukaryotic chromosome. Nature, Lond. 253, 245-249. BLOOM, W. & FAWCETT, D. W. (1968). A Textbook of Histology, 9th edn. Philadelphia, London and Toronto: Saunders. BOUTEILLE, M., LAVAL, M. & DUPUY-COIN, A. M. (1974). Localisation of nuclear functions as revealed by ultrastructural autoradiography and cytochemistry. In The Cell Nucleus, vol. 1 (ed. H. Busch), pp. 3-71. New York, London: Academic Press. BRUNS, R. R. & PALADE, G. E. (1968). Studies on blood capillaries. I. General organisation of blood capillaries in muscle. J. Cell Biol. 37, 244-276. BURGOYNE, L., HEWISH, D. & MOBBS, J. (1974). Mammalian chromatin substructure studies with the calcium-magnesium endonuclease and two-dimensional polyacrylamide-gel electrophoresis. Biochem. J. 143, 67-72. DAS, N . K., MICOU-EASTWOOD, J., RAMAMURTHY, G. & ALFERT, M. (1970). Sites of synthesis and processing of ribosomal RNA within the nucleolus of Urediis caupo eggs. Proc. natn. Acad. Sci. U.S.A. 67, 968-975. DAVIES, H. G. (1968). Electron-microscope observations on the organization of heterochromatin in certain cells J. Cell. Sci. 3, 129-150. DAVIES, H. G. (1976). Electron microscopy of interphase chromosomes in situ: binding of permanganate to chicken erythrocytes. J. Cell Sci. 20, 289-307. DAVIES, H. G. & HAYNES, M. E. (1975). Light- and electron-microscope observations on certain leukocytes in a teleost fish and a comparison of the envelope-limited monolayers of chromatin structural units in different species. J. Cell Sci. 17, 263-285. DAVIES, H. G., MURRAY, A. B. & WALMSLEY, M. E. (1974). Electron-microscope observations on the organization of the nucleus in chicken erythrocyte and a superunit thread hypothesis for chromosome structure. J. Cell Sci. 16, 261-299. DAVIES, H. G. & SMALL, J. V. (1968). Structural units in chromatin and their orientation on membranes. Nature, Lond. 217, 1122-1125. DAVIES, H. G. & TOOZE, J. (1966). Electron- and light-microscope observations on the spleen of the newt Triturus cristatus: the surface topography of the mitotic chromosomes. J. Cell Sci. 1, 331-35°EVERID, A. C , SMALL, J. V. & DAVIES, H. G. (1970). Electron-microscope observations on the structure of condensed chromatin: evidence for orderly arrays of unit threads on the surface of chicken erythrocyte nuclei. J. Cell Sci. 7, 35-48. HAYNES, M. E. & DAVIES, H. G. (1973). Observations on the origin and significance of the nuclear envelope-limited monolayers of chromatin unit threads associated with the cell nucleus. J. Cell Sci. 13, 139-171. HEWISH, D. R. & BURGOYNE, L. A. (1973). Chromatin sub-structure. The digestion of chromatin DNA at regularly spaced sites by a nuclear deoxyribonuclease. Biochem. biophys. Res. Commun. 52, 504-510. JEZEQUEL, A.-M., SHREEVE, M . M . &STEINER, J.W. (1967). Segregation of nucleolar components in Myco/>/ajma-infected cells. Lab. Invest. 16, 287-304. KORNBERG, R. D. (1974). Chromatin structure: a repeating unit of histones and DNA. Science, N.Y. 184, 868-871. KORNBERG, R. D. & THOMAS, J. O. (1974). Chromatin structure: oligomers of the histones. Science, N.Y. 184, 865-868. MACGREGOR, H. C. (1967). Pattern of incorporation of ['HJuridine into RNA of amphibian oocyte nucleoli. J. Cell Sci. 2, 145-150. MILLONIG, G. (1961). Advantages of phosphate buffer for osmium tetroxide solutions in fixation. J. appl. Phys. 32, 1637. Organization of interphase nuclei 327 NOLL, M. (1974). Subunit structure of chromatin. Nature, Lond. 251, 249-251. OLINS, A. L., CARLSON, R. D. & OLINS, D. E. (1975). Visualisation of chromatin substructure: ^-bodies. J. Cell Biol. 64, 528-537. OLINS, A. L. & OLINS, D. E. (1973). Spheroid chromatin units (e-bodies). J. Cell Biol. 59, 252a. OUDET, P., GROSS-BELLARD, M. & CHAMBON, P. (1975). Electron microscopic and biochemical evidence that chromatin structure is a repeating unit. Cell 4, 281-300. REYNOLDS, E. S. (1963). The use of lead citrate at high pH as an electron-opaque stain in electron microscopy. J. Cell Biol. 17, 208-212. RHODIN, J. A. G. (1967). The ultrastructure of mammalian arterioles and precapillary sphincters. J. Ultrastruct. Res. 18, 181-223. Ris, H. (1975). Chromosomal structure as seen by electron microscopy. In Ciba Fdn Symp. 28, The Structure and Function of Chromatin (ed. D. W. Fitzsimmons & G. E. W. Wolstenholme), pp. 7—28. Amsterdam: Elsevier. RlS, H. & KUBAI, D. F. (1970). Chromosome structure. A. Rev. Genet. 4, 263-294. ROBISON, W. G. & LIPTON, B. H. (1969). Advantages of dichromate-acrolein fixation for preservation of ultrastructural detail, jf. Cell Biol. 43, 117a. SMETANA, K. & BUSCH, H. (1974). The nucleolus and nucleolar DNA. In The Cell Nucleus, vol. 1 (ed. H. Busch), pp. 73-147. New York and London: Academic Press. SOLARI, A. J. (1974). The molecular organization of the chromatin fibre. In The Cell Nucleus, vol. 1 (ed. H. Busch), pp. 493-535. New York and London: Academic Press. SOLLNER-WEBB, B. & FELSENFELD, G. (1975). A comparison of the digestion of nuclei and chromatin by staphylococcal nuclease. Biochemistry, N.Y. 14, 2915-2920. SOLOFF, B. L. (1973). Buffered potassium permanganate-uranyl acetate—lead citrate staining sequence for ultrathin sections. Stain Technol. 48, 159—165. THOMAS, J. O. & KORNBERG, R. D. (1975). An octamer of histones in chromatin and free in solution. Proc. natn. Acad. Set. U.S.A. 73, 2626-2630. TOKUYASU, K., MADDEN, S. C. & ZELDIS, L. J. (1968). Fine structural alterations of interphase nuclei of lymphocytes stimulated to growth activity in vitro. J. Cell Biol. 39, 630-660. VAN HOLDE, K. E., SAHASRABUDDHE, C. G. & SHAW, B. R. (1974). A model for particulate structure in chromatin. Nucleic Acids Res. 1, 1579-1585. WALMSLEY, M. E. & DAVIES, H. G. (1975). Ultrastructural and biochemical observations on interphase nuclei isolated from chicken erythrocytes. J. Cell Set. 17, 113-139. WEINREB, E. L. & BILSTAD, N. M. (1955). Hjstology of the digestive tract and adjacent structures of the rainbow trout, Salmo gairdneri irideus. Copeia no. 3, 194—204. WOODCOCK, C. L. F. (1973). Ultrastructure of inactive chromatin. J. Cell Biol. 59, 368a. WOODCOCK, C. L. F., FRADO, L-L. Y. & SWEETMAN, H. E. (1975). Organisation of chromatin subunits. J. Cell Biol. 76, 462 a. ZIRKIN, B. R. & KIM, SUN-KEE. (1972). Fine structure of fibers in thin sections of condensed chromatin. Expl Cell Res. 75, 490—496. ZIRKIN, B. R. & WOLFE, S. L. (1972). Fiber ultrastructure and dimensions in thin-sectioned chromatin. J. Ultrastruct. Res. 39, 496-508. (Received 29 December 1975)