* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download Andrew Tibbits
Survey
Document related concepts
Transcript
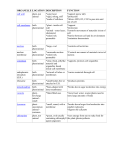
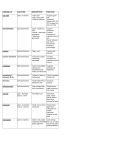
Photoinitiated “Bottom-Up” Click Synthesis of Ion-Containing Networks as Hydroxide Exchange Membranes Andrew C. Tibbits Advisors: Dr. Christopher Kloxin and Dr. Yushan Yan Committee: Dr. Norman Wagner, Dr. Bingjun Xu, and Dr. Darrin Pochan Fuel cells are energy conversion devices which directly convert chemical energy into electrical energy and environmentally friendly byproducts (i.e., water) with potential versatility for transportation and portable applications. Hydroxide exchange membrane fuel cells (HEMFCs) have the potential to decrease the overall fuel cell cost through the utilization of nonprecious metal catalysts such as nickel and silver as opposed to platinum which is used by the current standard technology, proton exchange membrane fuel cells (PEMFCs). However, substantial improvements in thermal and alkaline stability, hydroxide conductivity, mechanical flexibility, and processing are needed to create a competitive membrane for HEMFC applications. Regardless of the type of membrane, the high water uptake that is typically associated with increased ionic conductivity is problematic and can result in the dissolution of the membrane during fuel cell operation. Covalent crosslinking of the membrane is an approach which has been effectively applied to reduce water uptake without a significant compromise of the hydroxide conductivity. The synthesis and processing of membrane materials is vastly simplified by using click polymerization schemes. Click chemistry is a collection of organic chemical reactions that are rapid, selective, and high yielding. One of the most versatile and facile click reactions is the thiol-ene reaction, which is the radical-mediated addition reaction between a thiol (an –SH group) and an ‘ene’ (an electron rich vinyl group, C=C) in the presence of a photoinitiator and light. The click attributes of the thiol-ene reaction enable potential of “bottom-up” design of ion- containing polymers via photoinitiated crosslinking reaction in a single step with precise control over structure and physicochemical properties not only for fuel cell membranes but also for a range of other applications including separation membranes, sensors, flexible electronics, and coatings. However, a fundamental understanding of the formation and properties of ion- containing thiol-ene materials and their implementation as hydroxide exchange membranes is largely absent from the current literature. The work described herein will highlight the versatility of click reactions, primarily the thiol-ene reaction, for fabrication of ion-containing networks with tunable properties based on the rational design and synthesis of photopolymerizable ionic liquid comonomers with an emphasis on applicability for HEMFC applications. The role of ionic liquid monomer structure on the kinetics and mechanism of thiol-ene ionic network formation and the subsequent properties (i.e., ion conductive, thermomechanical, and structural) will be elucidated to establish a guided framework for click ionic material development. This framework will be directed onto the development of alkaline stable hydroxide-conductive membranes for fuel cell applications as well as the incorporation of catalytic nanoparticles into a photocrosslinkable formulation as a self-standing catalyst layer. Finally, novel approaches to membrane fabrication will be implemented to build on the foundational studies that will simultaneously enhance the ionic conductivity and mechanical properties of the ion-containing polymer materials: these approaches include the synthesis and crosslinking of photopolymerizable cationic surfactants for microphase separated membranes as well as the first “bottom-up” ion-containing polymer synthesized from the photoinitiated copper-catalyzed azide-alkyne cycloaddition (photoCuAAC) reaction which exhibits enhanced processability and hydroxide conductivity (>50 mS/cm).