* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download Pain-management-1-23-13
Prescription costs wikipedia , lookup
Discovery and development of direct thrombin inhibitors wikipedia , lookup
Drug design wikipedia , lookup
Pharmaceutical industry wikipedia , lookup
Discovery and development of cyclooxygenase 2 inhibitors wikipedia , lookup
NK1 receptor antagonist wikipedia , lookup
Psychopharmacology wikipedia , lookup
Atypical antipsychotic wikipedia , lookup
Drug discovery wikipedia , lookup
Pharmacokinetics wikipedia , lookup
Drug interaction wikipedia , lookup
Neuropsychopharmacology wikipedia , lookup
Neuropharmacology wikipedia , lookup
Pharmacognosy wikipedia , lookup
Theralizumab wikipedia , lookup
Polysubstance dependence wikipedia , lookup
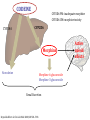
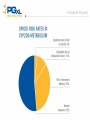
PGX Applications in Pain Management Kristen K. Reynolds, PhD VP Laboratory Operations Copyright 2012-2013 PGXL Laboratories, Louisville KY All materials herein are the exclusive property of PGXL Laboratories Panels* Core: Panel Add-Ons: CYP2D6 CYP2C9 CYP2C19 CYP3A4 CYP3A5 CYP1A2 OPRM1 (opioids) *All genes always orderable a la carte SLC6A4 (SSRIs) Common pain medications with PGXL tests **prodrug; + Generic Brand Metabolic Route Alfentanil Carisoprodol** Celecoxib Codeine** Cyclobenzaprine Fentanyl Hydrocodone** Hydromorphone Alfenta Soma Celebrex Various brands Flexaril Actiq, Duragesic Lortab, Vicodin Dilaudid CYP3A4/CYP3A5 CYP2C19 CYP2C9 CYP2D6 CYP1A2, CYP3A4/CYP3A5 CYP3A4/CYP3A5 CYP2D6 UGT2B7+ Ibuprofen Advil, Motrin CYP2C9 Lidocaine Methadone Morphine Naproxen Oxycodone** Oxymorphone Ropivicaine Tizanidine Tramadol** Zolmipitran Various brands Various brands Various brands Aleve Oxycontin, Percocet Opana Various brands Zanaflex Ultram, various Zomig CYP1A2 CYP2C19, CYP2B6+ UGT2B7+ CYP2C9 CYP2D6, CYP3A4/5 UGT2B7+ CYP1A2 CYP1A2 CYP2D6 CYP1A2 test not yet available Opioids Pharmacokinetic Gene Metabolism Pharmacodynamic Gene Clinical Effect CYP2D6 - Opioids Hydrocodone Oxycodone Codeine Propoxyphene Tramadol etc… CODEINE CYP2D6 PM: inadequate morphine CYP2D6 UM: morphine toxicity CYP3A4 CYP2D6 Morphine Norcodeine Morphine-6-glucuronide Morphine-3-glucuronide Renal Excretion Reynolds KR et al. Clin Lab Med 2008;28:581–598. Active opioid effects Effects of CYP2D6 Decreased drug metabolism = lack of efficacy – Poor pain control – Mis-interpretation of drug seeking behavior Ultra-rapid drug metabolism = possible side effects – Over-production of active compound – Mis-interpretation of over-compliance – Possible lower doses required Morphine Overdose from Codeine 8-15-12 FDA Drug Safety Codeine use in certain children after tonsillectomy and/or adenoidectomy may lead to rare, but life-threatening adverse events or death • 3 deaths reported in children (2-5yo) who received codeine after undergoing tonsillectomy and/or adenoidectomy for obstructive sleep apnea • 3 deaths in children who were CYP2D6 UMs • All children received typical codeine doses • Morphine toxicity signs developed within 1-2 days after starting codeine • Supratherpeutic post-mortem morphine concentrations in the 3 death cases FDA recommendations for Physicians: • • Use the lowest effective codeine dose for the shortest period of time on an as-needed basis (i.e., not scheduled around the clock) Counsel parents: – how to recognize the signs of morphine toxicity – Advise them to stop giving the child codeine – Seek medical attention immediately if child exhibits these signs • • Tests are available for determining CYP2D6 genotype Consider prescribing alternative analgesics for children CYP2D6 *4/*4 CYP2D6 Phenotype THERAPEUTIC IMPLICATIONS (adapted from published resources) Poor Metabolizer Avoid Alternative Consideration Adjust Dosage Adjustment Codeine** Hydrocodone** Oxycodone** Tramadol** Tamoxifen** Amitriptyline † Venlafaxine † Risperidone † Morphine, non-opioid Hydromorphone, non-opioid Oxymorphone, non-opioid Consider active drug, non-opioid Anastrozole, exemestane, letrozole Citalopram, sertraline Citalopram, sertraline Quetiapine, olanzapine, clozapine Aripiprazole † Clomipramine † Doxepin † Flecainide † Haloperidol † Imipramine† Nortriptyline † Propafenone † Metoprolol † 10 mg/day maximum decrease 50% decrease 60% decrease 50% decrease 50% decrease 70% decrease 60% decrease 70% decrease 75%, or atenolol, bisoprolol, carvedilol decrease 50%, or flupenthixol, quetiapine, olanzapine, clozapine Zuclopenthixol † **Lack of efficacy due to failure to produce active metabolite; †Increased risk of adverse events due to diminished drug clearance. CYP2D6 Poor Metabolizer (PM): This patient’s genotype is consistent with a lack of CYP2D6 enzymatic activity. PMs are at increased risk of drug-induced side effects due to diminished drug elimination of active drugs or lack of therapeutic effect resulting from failure to generate the active form of the drug, as is the case with pro-drugs. CONFIDENTIAL COPYRIGHT PGXL LABORATORIES 2012 RESULTS Gene X CYP2D6 *4/*4 THERAPEUTIC IMPLICATIONS (adapted from published resources) Phenotype Poor Metabolizer Avoid Codeine* Hydrocodone* Oxycodone* Tramadol* Normal metabolic clearance expected. Adjustment 10 mg/day maximum 50% 60% 50% 50% 70% 60% 70% 75%, or atenolol, bisoprolol, carvedilol † Zuclopenthixol 50%, or flupenthixol, quetiapine, olanzapine, clozapine Common CYP2D6 medications next page Oxycodone* Hydrocodone* † Propafenone Oxymorphone, non-opioid Hydromorphone, non-opioid Sotalol, disopyramide, quinidine, amiodarone Quetiapine, olanzapine, clozapine Citalopram, sertraline Codeine* Tramadol* Tamoxifen* Morphine, non-opioid Hydromorphone, non-opioid Oxymorphone, non-opioid Citalopram, sertraline Citalopram, sertraline Citalopram, sertraline Methylphenidate Quetiapine, olanzapine, clozapine Flupenthixol, quetiapine, olanzapine, clozapine Sotalol, disopyramide, quinidine, amiodarone Tramadol* † Imipramine † Nortriptyline † Venlafaxine Tamoxifen* Amitriptyline † Venlafaxine † Risperidone CYP2D6 *1/*1 ! CYP2D6 *1/*4 Extensive Metabolizer Intermediate Metabolizer Risperidone † † † Velafaxine X CYP2D6 *1/*1xN Ultra-Rapid Metabolizer Codeine* Hydrocodone* Oxycodone* † Amitriptyline † Clomipramine † Paroxetine † Atomoxetine † Risperidone Zuclopenthixol Propafenone † † Alternative Consideration Morphine, non-opioid Hydromorphone, non-opioid Oxymorphone, non-opioid Consider active drug, nonopioid Anastrozole, exemestane, letrozole Citalopram, sertraline Citalopram, sertraline Quetiapine, olanzapine, clozapine Adjust Dosage † Aripiprazole † Clomipramine † Doxepin † Flecainide † Haloperidol † Imipramine † Nortriptyline † Propafenone † Metoprolol † Amitriptyline † Imipramine † Nortriptyline † Zuclopenthixol † Doxepin † Flecainide † Metoprolol Haloperidol † † Doxepin † Metoprolol 15-60 mg/hr titrate to pain relief Avoid CYP2D6 inhibitors, e.g. paroxetine, or consider aromatase inhibitor in postmenopausal women 25% 30% 40% 25% 20% 25% 50%, or atenolol, bisoprolol, carvedilol 30% 70% 60% 150%, or citalopram, sertraline based on plasma measurement, or pimozide, flupenthixol, fluphenazine, quetiapine, olanzapine, clozapine 100% up to 250%, or atenolol, bisoprolol, carvedilol Pharmacokinetic Gene Metabolism Pharmacodynamic Gene Clinical Effect OPRM1: Mu Opioid Receptor Analgesia Sedation Euphoria Respiratory depression Itching Morphine Mu opioid receptor OPRM1 118A>G Genotypes AG AA GG Mean effective analgesic concentration = Therapeutic target range OPRM1: Mu Opioid Receptor 250 Opioid binds OPRM1 to elicit pain relief 200 Morphine mg/24hr 150 100 50 0 AA AG GG 118A>G variant decreases receptor availability and may increase dose requirements Reynolds 2008; Reyes-Gibby 2007; Klepstad 2004 OPRM1 helps predict dose of active opioids Morphine Hydromorphone Oxymorphone Generic Brand Metabolic Route Receptor/Dose Alfentanil Carisoprodol** Celecoxib Codeine** Cyclobenzaprine Alfenta Soma Celebrex Various brands Flexaril Fentanyl Hydrocodone** Hydromorphone Actiq, Duragesic Various brands Dilaudid CYP3A4/CYP3A5 CYP2C19 CYP2C9 CYP2D6 CYP1A2, CYP3A4/CYP3A5 CYP3A4/CYP3A5 CYP2D6 UGT2B7+ OPRM1 Ibuprofen Advil, Motrin CYP2C9 Lidocaine Methadone Morphine Naproxen Oxycodone** Oxymorphone Ropivicaine Tizanidine Tramadol** Zolmipitran Various brands Various brands Various brands Aleve Oxycontin, various Opana Various brands Zanaflex Ultram, various Zomig CYP1A2 CYP2C19, CYP2B6+ UGT2B7+ CYP2C9 CYP2D6, CYP3A4/5 UGT2B7+ CYP1A2 CYP1A2 CYP2D6 CYP1A2 **prodrug; + test not yet available OPRM1 OPRM1 OPRM1 Opioid Interpretations OPRM1 AA OPRM1 Phenotype Therapeutic Implications (adapted from published resources) Normal Opioid Opioid response: Average doses of morphine typically required (may also apply to other active opioids, eg, Responder hydromorphone, oxymorphone). Note: Formation of active opioid metabolites (e.g., morphine) from prodrugs (eg, codeine) is dependent on CYP2D6 activity. OPRM1 AG OPRM1 Phenotype Intermediate Opioid Responder OPRM1 GG OPRM1 Phenotype Poor Opioid Responder Therapeutic Implications (adapted from published resources) Opioid response: Higher then average doses of morphine typically required (may also apply to other active opioids, eg, hydromorphone, oxymorphone). Note: Formation of active opioid metabolites (e.g., morphine) from prodrugs (eg, codeine) is dependent on CYP2D6 activity. Adjust Dosage Adjustment Morphine Increase 10% Therapeutic Implications (adapted from published resources) Opioid response: Higher then average doses of morphine typically required (may Adjust Dosage Adjustment also apply to other active opioids, eg, hydromorphone, oxymorphone). Note: Morphine Increase up to Formation of active opioid metabolites (e.g., morphine) from prodrugs (eg, 80% codeine) is dependent on CYP2D6 activity. OPRM1 and Naltrexone for Alcohol dependence OPRM1 and Naltrexone Naltrexone is a competitive mu opioid receptor antagonist – Decreases alcohol cravings – Inhibits endorphin “reward” effects in EtOH/opioid abuse – Decreases relapse risk EtOH OPRM1 and risk of alcohol relapse • 40% of patients treated with naltrexone will relapse • 80% of those who relapse have AA genotype Chamorro et al. Addiction Biology 2012;17:505-12. OPRM1 AA carriers treated with naltrexone have highest risk of relapse AA genotype pts are 2x more likely to relapse that G carriers Chamorro et al. Addiction Biology 2012;17:505-12. OPRM1 Combined Interpretations OPRM1 AA OPRM1 Phenotype Therapeutic Implications (adapted from published resources) Normal Opioid Opioid response: Average doses of morphine typically required (may also apply to other active opioids, eg, Responder / hydromorphone, oxymorphone). Note: Formation of active opioid metabolites (e.g., morphine) from Impaired prodrugs (eg, codeine) is dependent on CYP2D6 activity. Naltrexone Responder Naltrexone response: With respect to naltrexone treatment for alcohol dependence, 80% of treated patients who relapse have the OPRM1 AA genotype. Relapse rate among AA genotype patients is 5% higher than the typical on-treatment relapse rate, and is 2-fold greater than for patients with the AG or GG genotypes. OPRM1 Combined Interpretations OPRM1 GG OPRM1 Phenotype Poor Opioid Responder / Normal Naltrexone Responder Therapeutic Implications (adapted from published resources) Opioid response: Higher then average doses of morphine typically required (may also Adjust Dosage apply to other active opioids, eg, hydromorphone, oxymorphone). Note: Formation of Morphine active opioid metabolites (e.g., morphine) from prodrugs (eg, codeine) is dependent on CYP2D6 activity. Naltrexone response: In patients treated with naltrexone for alcohol dependence, those with the GG genotype have a 15% lower average relapse rate compared to the typical on-treatment relapse rate. Overall, relapse rate among G allele carriers is 25% compared to 54% in patients with AA genotype. Adjustment Increase up to 80% OPRM1 Combined Interpretations OPRM1 GG OPRM1 Phenotype Poor Opioid Responder / Normal Naltrexone Responder Therapeutic Implications (adapted from published resources) Opioid response: Higher then average doses of morphine typically required (may also Adjust Dosage apply to other active opioids, eg, hydromorphone, oxymorphone). Note: Formation of Morphine active opioid metabolites (e.g., morphine) from prodrugs (eg, codeine) is dependent on CYP2D6 activity. Naltrexone response: In patients treated with naltrexone for alcohol dependence, those with the GG genotype have a 15% lower average relapse rate compared to the typical on-treatment relapse rate. Overall, relapse rate among G allele carriers is 25% compared to 54% in patients with AA genotype. Adjustment Increase up to 80% NSAIDs NSAIDs • • • Non-steroidal anti-inflammatory drugs Ibuprofen, naproxen, celecoxib, diclofenac, etc Individual or combination preparations • Celebrex (celecoxib) • Vicoprofen (hydrocodone + ibuprofen) NSAID Adverse Events Severe ADRs • Ulcer • GI bleed • Kidney failure • MI • Stroke Metabolized by CYP2C9 CYP2C9 variants increase risk of NSAID-induced bleeding • Case control study: 26 patients taking NSAID <1 month with confirmed GI bleed vs 52 NSAID users without GI bleeds • CYP2C9 genotyping performed CYP2C9 Genotype Risk of GI bleed taking NSAID *1/*1 1 *1/*2 3.8 *1/*3 7.3 Pilotto et al Gastroenterology 2007;133(2):465-71. CYP2C9 and NSAID use • CYP2C9 genotyping may identify patient subgroups at increased risk of NSAID-related GI bleeding • Use with caution based on other clinical factors Summary Pearls • • • • • Opioid prodrug efficacy/ADR: 2D6 Active opioid dose: OPRM1 Naltrexone efficacy: OPRM1 NSAID ADR: 2C9 Other opioids and muscle relaxers: 3A4/5, 2C19, 1A2