* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download Agenus JP Morgan 2017 1-6
Survey
Document related concepts
Transcript
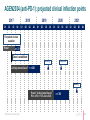
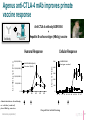
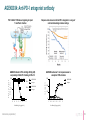
January 2017 Forward-looking statements This presentation contains forward-looking statements. These forward-looking statements are subject to risks and uncertainties, including the factors described under the Risk Factors section of our Quarterly Report on form 10-Q filed with the Securities and Exchange Commission on November 9, 2016 and made available on our website at www.agenusbio.com. When evaluating Agenus’ business and prospects, careful consideration should be given to these risks and uncertainties. These statements speak only as of the date of this presentation, and Agenus undertakes no obligation to update or revise these statements. This presentation and the information contained herein do not constitute an offer or solicitation of an offer for sale of any securities. 2 Broad I-O portfolio: enabling optimal combos 3 Broad I-O portfolio: opportunistic 4 Agenus Five Year Growth Plan: balancing low & high risk initiatives • Advance on most rapid path to BLA • • Develop, register, and launch anti-PD-1/CTLA-4 mAb combinations in validated indications Pursue novel breakthrough indications to expedite market entry • Leverage novel targets for market expansion • Pursue optimal I-O mAb and vaccine combinations with anti-CTLA-4 +/- anti-PD-1 • Progress partnered programs (GITR, OX40, TIM-3, LAG-3) towards registration • Advance Ab programs against innovative undisclosed targets to the clinic • Engage in strategic partnerships 5 Current partnerships • QS-21 Stimulon®: adjuvant for Shingrix® • GSK filed for registration in U.S., Canada and Europe • Royalties and milestones partly monetized • 1 undisclosed target • Lead selection completed • Up to $100 million in milestones • • • • Pre-clinical: LAG-3, TIM-3, 3 undisclosed Clinical: GITR, OX40 50/50 cost and profit share* Royalty rates are generally 6-12 %** and up to $350 million in milestones across the 4 programs * Specific to the OX40, GITR and two undisclosed programs ** Specific to the TIM-3, LAG-3 and one undisclosed program 6 Clinical Strategy New clinical development paradigm compressed Risk AGEN1884 / AGEN2034 + Novel CPM (A, B, C) AGEN1884 / AGEN2034 + Vaccine AGEN1884 / AGEN2034 Solid Tumors Efficacy unknown AGEN1884 / AGEN2034 Solid Tumors Efficacy proven AGEN1884 = anti-CTLA-4 mAb AGEN2034 = anti-PD-1 mAb CPM = checkpoint modulator 10 years Conventional time to BLA 8 anti-CTLA-4 + anti-PD-1: first clinically validated I-O mAb combo • anti-CTLA-4 (low dose) combined with anti-PD-1/PD-L1 is the only validated mAb combination with improved efficacy and safety profile • Near doubling of clinical response - from ~25% to 40% in NSCLC(1,2) • Control of CTLA-4 + PD-1/PD-L1 targeted therapies could offer a pricing advantage • Provides a foundation for mAbs against novel checkpoints that are yet to show efficacy in the absence of CTLA-4 antagonism 1. 2016 ASCO Annual Meeting; CheckMate -012 study; abstract no. 3001 2. BMS press release 06/04/2016 *Anti-CTLA-4 antibody AGEN1884 is partnered with Recepta for certain South American territories 9 Targeting PD-1 has clinical benefit but combining with CTLA-4 antagonism works better • anti-CTLA-4 (low dose) combination with anti-PD-1/PD-L1 reduces development risk and expands markets • anti-CTLA-4 and anti-PD-1 mAb combination is already approved in metastatic melanoma & almost doubles clinical benefit in 1L NSCLC(1,2) • CTLA-4 + PD-1/PD-L1 antagonists +/- mAbs targeting novel checkpoints have shown compelling data in preclinical models 1. 2016 ASCO Annual Meeting; CheckMate -012 study; abstract no. 3001 2. BMS press release 06/04/2016 *Anti-CTLA-4 antibody AGEN1884 is partnered with Recepta for certain South American territories 10 Metastatic virally induced malignancies*: appealing target for Agenus • Clinical development: • SOC marginally changed after the approval of Avastin in combination with chemo in 1L • No effective treatment options in 2L • Clinical activity of other anti PD-X in virally induced and HPV driven malignancy (HNSCC HPV+) suggests that AGEN1884 administration could lead to an ORR ~ 15% in all comers, > 20% in PD-L1+ •Regulatory: • US: Possibility of applying for Breakthrough Designation, assuming hypotheses are backed up by clinical data, Accelerated Approval possible • EU: Possibility of applying for conditional marketing authorization • Commercial: • Potential niche opportunity with most of the patients from Japan and South Korea where premium prices for unmet medical needs are commonly given and where there is no off-label use reimbursed * Anticipated in cervical cancer 11 AGEN2034 (anti-PD-1): projected clinical inflection points 2017 Q1 Q2 Q3 2018 Q4 Q1 Q2 Q3 2019 Q4 Q1 Q2 Q3 2020 Q4 Q1 Q2 Q3 2021 Q4 Q1 Q2 Q3 Q4 First patient for dose escalation Phase 1 n = 30 Dose is established US filing US approval * *n = 200 2L Virally-induced Cancer US filing ** Phase 3 Virally-induced Cancer PD-1 vs PD-1 + CTLA-4 vs SOC * Anticipated in cervical cancer n = 700 12 AGEN2034 for 2L advanced virally induced malignancy: potential value • Could provide a unique registration opportunity for one of our agents as a monotherapy within the next 5 years • Unmet medical need • Opportunity to validate the PD-1 element of our PD-1/CTLA-4 plans • Establish commercial presence in ~ 4 years of development • Multiple regulatory designations possible including Sakigake and Breakthrough Designation that could provide external government issued program validation • Acceptable technical and regulatory risk 13 Preclinical data Shaping the immune response to cancer: targeting coordinated nodes of immune regulation * Antibodies partnered with Incyte: GITR, OX40 (agonists);TIM3, LAG3 (antagonists) and three undisclosed targets ** Antibodies partnered with Recepta for certain South American territories ^^ ASV: AutoSynVax™, PSV: PhosphoSynVax™ 15 Integrated antibody discovery technologies, combined with immunology expertise 16 Agenus anti-CTLA-4 mAb improves primate vaccine response Antibody Anti-CTLA-4 antibody AGEN1884 + Hepatitis B surface antigen (HBsAg) vaccine Vaccine Cellular Response 2 ,0 0 0 ,0 0 0 Is o ty p e c o n tro l C o n tr o l (N = 6 ) 400 300 6 1 ,5 0 0 ,0 0 0 AG EN1884 500 A G E N 1 8 8 4 (N = 6 ) S F U /1 x 1 0 P B M C A n ti-H B s A g Ig G (U /m L ) Humoral Response 1 ,0 0 0 ,0 0 0 5 0 0 ,0 0 0 0 200 100 0 -7 15 29 43 59 69 -4 0 8 15 22 29 36 43 50 57 67 A d m in is tra tio n s o f a n tib o d y o r v e h ic le (c o n tr o l) p lu s H B s A g v a c c in e Internal data (unpublished) D a y s A fte r In itia l D o s in g 17 AGEN2034: Anti-PD-1 antagonist antibody PD-1 inhibits TCR-induced signaling to impair T cell effector function Response rates observed with an PD-1 antagonist in a range of solid and hematological tumor settings AGEN2034 binds to PD-1 with high affinity (nM) and potently inhibits PD-1 binding to PD-L1/2 P D -L 2 50 300 A G EN 2034 is o t y p e A G EN 2034 is o t y p e IL - 2 ( p g /m l) P D -L 1 b in d in g ( % ) P D -L 1 o r P D -L 2 100 AGEN2034 enhances T cell responsiveness to suboptimal TCR activation A G EN 2034 I s o ty p e 200 100 0 0 -5 -4 -3 -2 -1 0 1 A n t ib o d y ( lo g µ g / m L ) Internal data (unpublished) 2 -7 -6 -5 -4 -3 -2 -1 0 1 2 A n t ib o d y ( lo g µ g / m L ) 18 AGEN1884 combines in primary T cell assays with PD-1:PD-L1 blockade (as well as LAG-3) Isotype Pembrolizumab Internal data (unpublished) 19 Business Projected milestones 2016 2017 2018 Accomplishments Clinical Deliverables Clinical Activity & Readouts • Three CPM mAbs in clinic • CTLA-4 antagonist (AGEN1884)* • GITR agonist (INCAGN1876)** • OX40 agonist (INCAGN1949)** • Clinical trials • Initiate Ph 1 for PD-1 antagonist (AGEN2034)* monotherapy • Initiate virally-induced cancer+ trial (2L) • Initiate Ph 1b with AGEN1884* + AGEN2034* • Initiate Ph 1 with AutoSynVax™ • Complete enrollment for 2nd line virally-induced cancer+ cohort • Clinical Development team with I-O development success on board • QS-21 Stimulon® containing Shingles vaccine filed for regulatory approval • Clinical results • Optimal monotherapy dose for AGEN2034* • Optimal combination dose of AGEN2034* + AGEN1884* • Clinical responses • Results from 30 patients in the virallyinduced cancer+ cohort (2L) • Initial immune biomarker data from 6patient Ph 1 ASV proof-of-mechanism trial • INCY collaboration expanded * Partnered with Recepta for certain South American rights ** Partnered with INCY + Anticipated in cervical cancer • Top line data for the virallyinduced cancer+ cohort: • Response rate • Duration of response • Safety and tolerability 21 Management Team • Garo Armen, PhD, Chairman & CEO Elan Corporation, plc, Protagenic Therapeutics, Founder - Children of Armenia Fund (COAF) • Robert Stein, MD, PhD, President, R&D Incyte Pharmaceuticals, Ligand Pharmaceuticals, Dupont/Merck, Roche, KineMed, Merck, Sharp & Dohme • Jennifer Buell, PhD, VP Research & Development Operations Harvard Clinical Research, Bristol-Myers Squibb • Christian Cortis, PhD, VP Business Development Synta Pharmaceuticals, Advanced Technology Ventures, Columbia University • Jean-Marie Cuillerot, MD, VP & Global Head of Clinical Development EMD Serono, Bristol-Myers Squibb, University Louis Pasteur • Alex Duncan, PhD, Chief Technology Officer Actigen, Affitech A/S, Astra Zeneca, Cambridge Antibody Technology • James Gorman, MD, PhD, VP Corporate Development and Strategy Abbott Laboratories, Harvard University • Christine Klaskin, VP Finance Arthur Andersen, George Washington University • Michelle Linn, VP Corporate Communications Linnden Communications, Ogilvy PR/Feinstein Kean, Chair of Women In Bio Boston Chapter • Karen Valentine, JD, Chief Legal Officer & General Counsel Palmer and Dodge LLP 22