* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download Chpt6 - Dr. Erdal ONURHAN
George S. Hammond wikipedia , lookup
Marcus theory wikipedia , lookup
X-ray photoelectron spectroscopy wikipedia , lookup
Rutherford backscattering spectrometry wikipedia , lookup
Heat transfer physics wikipedia , lookup
Transition state theory wikipedia , lookup
Physical organic chemistry wikipedia , lookup
Eigenstate thermalization hypothesis wikipedia , lookup
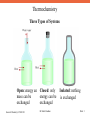
Thermochemistry Some Forms of Energy - Solar Energy, part of radiant energy. Solar energy comes from the Sun and is the primary source of Earth’s energy. - Thermal Energy is due to random motion of atoms and molecules. It is generally calculated from temperature measurements. Temperature is not a measure of thermal energy itself. - Chemical Energy is stored within the structural units of chemical substances. When a chemical reaction takes place, chemical energy is released, stored, or converted to other forms of energy. - Potential Energy is the energy due to the position of an object. Chemical energy can be considered to be a form of potential energy, since it is associated with the positions of atoms in the molecules. - Kinetic Energy is the energy produced by a moving object. General Chemistry I CHM 111 Dr Erdal Onurhan Slide 1 Thermochemistry System-Boundary-Surroundings The boundary might be imaginary, as in this case, or real, as in other cases. General Chemistry I CHM 111 Dr Erdal Onurhan Slide 2 Thermochemistry Three Types of Systems Open: energy an Closed: only mass can be energy can be exchanged exchanged General Chemistry I CHM 111 Dr Erdal Onurhan Isolated: nothing is exchanged Slide 3 Thermochemistry An Example to State Functions – Distance vs. Displacement There are many routes between A and B. The distance covered by the coloured routes is different from one another. The displacement, however, is the same for all routes, AB. Displacement is a state function, whereas distance is not. General Chemistry I CHM 111 Dr Erdal Onurhan Slide 4 Thermochemistry Another Example of a State Function- Volume General Chemistry I CHM 111 Dr Erdal Onurhan Slide 5 Thermochemistry The First Law of Thermodynamics It is obvious that, energy lost by the system is absorbed by the surroundings. Since the Universe comprises both the system and the surroundings, energy is conserved in the process. General Chemistry I CHM 111 Dr Erdal Onurhan Slide 6 Thermochemistry Sign Convention for Work and Heat General Chemistry I CHM 111 Dr Erdal Onurhan Slide 7 Thermochemistry Expansion of a Gas in a Cylinder Initial State General Chemistry I CHM 111 Final State Dr Erdal Onurhan Slide 8 Thermochemistry Bomb Calorimeter The thermometer is used to determine t of the reaction. qcal is calculated from qcal Ccal t General Chemistry I CHM 111 Dr Erdal Onurhan Slide 9 Thermochemistry An Open Calorimeter The thermometer is used to determine t of the reaction. q is calculated from q msT General Chemistry I CHM 111 Dr Erdal Onurhan Slide 10