* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download Development of Quantum Mechanics Bohr*s Contribution
Quantum electrodynamics wikipedia , lookup
Theoretical and experimental justification for the Schrödinger equation wikipedia , lookup
X-ray photoelectron spectroscopy wikipedia , lookup
James Franck wikipedia , lookup
Wave–particle duality wikipedia , lookup
Electron scattering wikipedia , lookup
X-ray fluorescence wikipedia , lookup
Tight binding wikipedia , lookup
Atomic orbital wikipedia , lookup
Bohr–Einstein debates wikipedia , lookup
Electron configuration wikipedia , lookup
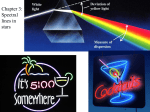
Development of Quantum Mechanics Bohr’s Contribution AP Chemistry by Diane Paskowski Questions to Consider Why do the different chemicals give us different colors? Why do we get colors at all? 7.1 Atomic Spectrum of Hydrogen Continuous spectrum – contains all the wavelengths of visible light Line spectrum – each line corresponds to a discrete wavelength: Hydrogen emission spectrum 7.3 Significance? Only certain energies are allowed for the electron in the hydrogen atom. Energy of the electron in the hydrogen atom is quantized. 7.3 Bohr Model Electron in a hydrogen atom moves around the nucleus only in certain allowed circular orbits. ΔE = -2.178×10-18J[(1/nfinal2)-(1/ninitial2)] Where n is the energy level (principal quantum number) 7.4 Electronic Transitions in the Bohr Model for the Hydrogen Atom 7.4 Figure 7.7 A Change Between Two Discrete Energy Levels Emits a Photon of Light Line Spectrum Of Hydrogen http://www.mhhe.com/physsci/chemistry/essentialchemis try/flash/linesp16.swf Bohr Model Bohr’s model is fundamentally incorrect. This model only works for hydrogen. Electrons do not move around the nucleus in circular orbits. 7.4