* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download Exploration of the Cause of the Low Intensity Aortic Component of
Cardiac contractility modulation wikipedia , lookup
Electrocardiography wikipedia , lookup
Coronary artery disease wikipedia , lookup
Myocardial infarction wikipedia , lookup
Mitral insufficiency wikipedia , lookup
Management of acute coronary syndrome wikipedia , lookup
Antihypertensive drug wikipedia , lookup
Jatene procedure wikipedia , lookup
Arrhythmogenic right ventricular dysplasia wikipedia , lookup
Hypertrophic cardiomyopathy wikipedia , lookup
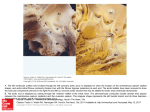
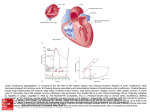
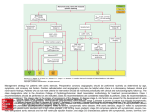
590 46. 47. 48. 49. CIRCULATION relations in failing and nonfailing hearts of subjects with aortic stenosis. Am J Med Sci 259: 79, 1970 Huggenholtz PG, Ellison C, Urschel CW, Mirskey I, Sonnenblick EH: Myocardial force-velocity relationships in clinical heart disease. Circulation 41: 191, 1970 Mehmel HC, Mazzoni S, Krayenbuehl HP: Contractility of the hypertrophied human left ventricle in chronic pressure and volume overload. Am Heart J 90: 236, 1975 Brutsaert DL, Paulus WJ: Loading and performance of the heart as muscle and pump. Circ Res 11: 1, 1977 Chandler BM, Sonnenblick EH, Spann JF Jr, Pool PE: Association of VOL 57, No 3, MARCH 1978 depressed myofibrillar adenosine triphosphatase and reduced contractility in experimental heart failure. Circ Res 21: 717, 1967 50. Spann JF Jr, Chidsey CA, Pool PE, Braunwald E: Mechanism of norepinephrine depletion in experimental heart failure produced by aortic constriction in the guinea pig. Circ Res 17: 312, 1965 51. Chidsey CA, Kaiser GA, Sonnenblick EH, Spann JF, Braunwald E: Cardiac norepinephrine stores in experimental heart failure in the dog. J Clin Invest 43: 2386, 1964 52. Buccino RA, Harris E, Spann JF, Sonnenblick EH: Response of myocardial connective tissue to development of experimental hypertrophy. Am J Physiol 216: 425, 1969 Exploration of the Cause of the Low Intensity Aortic Component of the Second Sound in Nonhypotensive Patients with Poor Ventricular Performance Downloaded from http://circ.ahajournals.org/ by guest on June 17, 2017 PAUL D. STEIN, M.D., HANI N. SABBAH, B.S., FAREED KHAJA, M.D., AND DANIEL T. ANBE, M.D. SUMMARY This investigation was undertaken to explore the cause of the diminished second sound (S2) that may occur in normotensive patients with poorly performing ventricles. Intraaortic sound and pressure were measured in 16 patients with angina; eight had normal ventricular performance (ejection fraction . 60%) and eight had poor performance (ejection fraction < 50%). The amplitude of S, was lower in patients with poor ventricular performance as was negative dp/dt. Aortic pressure was com- parable in both groups. The ampitude of S2 was linearly related to the rate of change of the pressure gradient that developed across the aortic valve during diastole (r = 0.82). The latter also correlated with negative dp/dt (r = 0.82). These observations indicate that in patients with poor ventricular performance, isovolumic relax- THE AORTIC COMPONENT of the second sound may be diminished in patients with myocardial infarction or congestive heart failure, even in the absence of a reduced blood pressure.13 Traditional teaching that considers the amplitude of the second sound to be primarily determined by diastolic pressure does not explain this observation.2' In order to explain these clinical observations, one must assume that factors other than diastolic pressure contribute to the intensity of the second sound. Other pressure related factors that have been suggested or shown to relate to the amplitude of the aortic component of the second sound include the diastolic pressure gradient that develops across the closed valve,6 the maximal rate of change of the diastolic pressure gradient7' 8 and the pressure gradient at the incisura.7 The rate of change of the diastolic pressure gradient correlates best with the amplitude of the second sound.7 8 The velocity of retrograde aortic flow9 and deceleration of flow'0 have also been suggested as factors which could affect the amplitude of the second sound. However, if one conceives of the second sound as being caused by vibration of the closed cusps"' 1' then it can be demonstrated by mathematical analysis of factors that would effect vibration, that the driving force productive of vibration is the diastolic pressure difference that develops across the valve.'3 The amplitude of sound that would result from such vibrations relates to the rate of change of that pressure difference.13 Neither retrograde flow nor the deceleration of flow were shown to be the forces productive of valvular vibration. Thus, it seems from previous studies that the rate of change of the pressure gradient that develops across the valve in diastole is a primary determinant of the amplitude of the second sound.'1-13 The purpose of this study is to explore the extent to which the rate of change of the diastolic pressure gradient, and factors which affect it, may participate in causing a diminished aortic component of the second sound which is sometimes observed in normotensive patients following a myocardial infarction or in heart failure. From the Departments of Medicine and Surgery, Henry Ford Hospital, Detroit, Michigan. Supported in part by grant #R38820 from funds supplied by Henry Ford Hospital via a Ford Foundation grant. Address for reprints: Paul D. Stein, M.D., Henry Ford Hospital, 2799 West Grand Boulevard, Detroit, Michigan 48202. Received September 8, 1977; revision accepted October 18, 1977. ation may be compromised. This would cause a reduction of the rate of development of the diastolic pressure gradient, which would result in a diminished S2. Methods Intra-aortic sound was measured during diagnostic cardiac catheterization in 16 patients with anginal-like pain. Three had no apparent cardiac disease, 12 had coronary heart disease, and one had cardiomyopathy. Eight patients had normal ventricular performance as judged by an ejection fraction of 60% or more; and eight patients had poor ventricular performances, indicated by an ejection fraction of less than 50%. One patient was excluded because he had an ejection fraction of 54% which may not be abnormal according to the criteria of some investigators,'4 yet is below the range of normal found by others." Patients were also ex- SECOND SOUND AND VENTRICULAR PERFORMANCE/Stein 1 40 Pressure and sound in all aorta and left ventricle tip cr C/) 0. 50r- z w a: / 40 cor 301- a: CD - 201- 0uJ, 00 CO Downloaded from http://circ.ahajournals.org/ by guest on June 17, 2017 Hn _- / LOPED(IP)/DT o Co0 . n] I - 10 20 30 40 50 TIME(MSEC) Top) Aortic (Ao) and left ventricular (LV) pressure (patient #16). The pressure gradient (Ap) that develops across the aortic valve during diastole is shown by vertical lines. Bottom) The diastolic pressure gradient, Ap, is shown as a function of time. The slope of the curve is the rate of change of the pressure gradient FIGURE 1. d(Ap)/dt. cluded if they had audible systolic or diastolic murmurs, or any diastolic murmur recorded within the left ventricle. None had pressure gradients measured across the aortic valve. All patients received sedation with diazepam (Valium) which was TABLE Patient taken orally. 1. Second Sound S Age 1 2 54 44 44 38 51 3 4 6 7 8 Mean +SEM 1 2 3 4 5 591 at5 patients were measured in the using a Millar Instruments catheter- micromanometer. In all but two patients a single catheter-tip sensor was used. Aortic and ventricular pressure and sound were measured in rapid sequence during pull- w w et 31 53 46 45 Sex M MF MF M M M 46 46 niques. Results The sound of theaortic component of the second lower in patients with poor ventricular perfor- amplitude was Amplitude and Related Hemodynamic and Clinical Data Diagnosis CAD NL NL CAD NL CAD CAD CAD Amplitude of sound (dynes/cm2) Aortic pressure (mm Hg) LVEDP (mm Hg) Max dp/dt (mm Hg/sec) (mmHg/sec) Max d(Ap) /dt M M M M MF CAD CAD CAD CAD CAD - Patients with normal ventricular performance 1620 2020 5860 9 137/90 2000 6 2170 5470 117/79 1430 1320 8 3080 111/63 1170 1290 4 2520 104/77 2430 1570 7 3280 134/76 1250 16 1190 1560 121/85 2070 2200 5 2900 136/85 1890 2020 3 4100 146/98 1730 7 1720 3600 126/82 160 160 1 5/4 Patients with Abnormal ventricular performance 1590 1560 19 3070 170/80 1090 22 1090 2060 145/78 1140 1270 7 2900 120/84 1040 14 1110 2360 106/69 920 22 750 1470 133/95 890 22 1180 1210 120/96 920 1160 10 2080 108/88 860 14 1010 1210 103/65 1060 1140 16 2050 126/82 80 2 80 250 8/4 <0.01 <0.01 <0.01 <0.02 520 3 59 61 41 back of the catheter across the aortic valve. The RR intervals of beats from which calculations were made were virtually the same in the aorta and left ventricle (0.82 ± 0.03 sec vs 0.81 ± 0.03 sec) (mean ± SEm). Aortic and ventricular pressure and sound were measured simultaneously in two patients using a dual catheter-tip micromanometer. The amplitude of the aortic component of the second sound was measured as the peak-to-peak amplitude of the largest deflection. It was calibrated in terms of pressure (dynes/cm') as previously described.'6 The characteristics of the sound transducer and recording system also were previously described.'5 Three consecutive beats were selected for detailed analysis. In those beats, instantaneous values of the aortic and left ventricular diastolic pressure were digitized at 0.25 mm (1.25 msec) intervals using an electronic hand held digitizer (Numonics Corp.) on line with a Hewlett-Packard 21 MX computer. The data were referenced to the electrocardiogram by superumposing the QRS complexes. Instantaneous values of the difference between the aortic and left ventricular diastolic pressure were calculated from the point at which a pressure difference was measurable until the point at which the pressure difference was nearly constant (fig. 1). Instantaneous values of the rate of change of the diastolic pressure gradient were determined by curve fitting tech- 6 45 Cardiomyopathy 46 F CAD 7 54 CAD 8 M 50 Mean 3 +SEM P value pressure gradient; d(Ap)/dt = rate of change of the diastolic pressure gradient. Key: Ap = diastolic P values refer to the difference of means between the two groups (unpaired i-test). Max ApHg) (mm 117 Max Ap at incisura (mm Hg) Ejection fraction (%) 71 67 60 60 88 4 33 13 23 9 62 33 20 15 26 6 105 99 93 46 25 17 76 94 88 95 81 91 3 18 4 8 6 9 17 47 44 43 19 88 88 82 99 100 108 11199 5 61 64 80 69 4 12 16 35 33 31 5 CIRCULATION 592 Downloaded from http://circ.ahajournals.org/ by guest on June 17, 2017 mance (2050 ± 250 dynes/cm2) than in patients with normal ventricular performance (3600 ± 520 dynes/cm2, P < 0.02, unpaired t-test) (table 1). Negative dp/dt was lower in those with poor performance (1060 ± 80 vs 1730 ± 160 mm Hg/sec, P < 0.01). The maximal rate of change of the diastolic pressure gradient was also lower in patients with poor ventricular performance (1140 + 80 vs 1720 ± 160 mm Hg/sec, P < 0.01). The amplitude of the aortic component of the second sound showed a linear correlation with the maximal rate of change of the diastolic pressure gradient that developed across the valve (r = 0.82) (fig. 2). Maximal values of the rate of change of the diastolic pressure gradient occurred coincident with the major component of the second sound. A linear correlation was shown between the rate of change of the diastolic pressure gradient and negative dp/dt (r = 0.82). Negative dp/dt correlated with the second sound (r = 0.68), but the correlation with sound was not as close as the rate of change of the pressure difference. The amplitude of the second sound also correlated with the ejection fraction (r = 0.67). Again the correlation was not as close as the correlation between sound and the rate of change of the pressure gradient. Aortic pressure was comparable in both groups (126/82 ± 8/4 vs 126/82 ± 5/4 mm Hg). The amplitude of the aortic component of the second sound showed a poor correlation with aortic diastolic pressure (r = 0.10); the aortic pressure at the time of the incisura (r = 0.28); the maximal value of the diastolic pressure gradient, (r = 0.47); and with the pressure gradient at the time of the aortic incisura (r= 0.28). Discussion Even though the aortic pressure of the patients with poor ventricular performance was virtually the same as the pressure of those with normal performance, the amplitude of the aortic component of the second sound was significantly diminished in those with poor performance. Both negative dp/dt and the rate of change of the diastolic pressure gradient were also lower in patients with poor performance. 6000 Y= 2.5X -818 O R=.82 O v 4000 Z 0~ Cl° _ Oz 02000- 0 400 800 1200 1600 2000 MAXIMAL RATE OF CHANGE OF DIASTOLIC PRESSURE GRADIENT (MM HGJSEC) FIGURE 2. Relation of the amplitude of the aortic component of the sound to the maximal rate of change of the diastolic pressure gradient. The regression equation and correlation coefficient (R) are shown. Open circles are patients with an ejection fraction < 50%. Closed circles are patients with an ejection fraction > 60%. VOL 57, No 3, MARCH 1978 A dependence of the amplitude of the aortic component of the second sound upon the rate of change of the diastolic pressure gradient is predicted by equations which describe sound produced by the aortic valve when the valve is modeled as a vibrating circular membrane.13 Since the rate of change of the diastolic pressure gradient is dependent upon negative dp/dt, the results imply a dependence of the amplitude of the aortic closure sound upon the rate of isovolumic relaxation. We have observed a diminished aortic component of the second sound on phonocardiograms taken at the chest wall in normotensive patients hospitalized because of recent ischemic episodes."7 In these patients, the duration of isovolumic relaxation was prolonged, which also implies a reduced rate of isovolumic relaxation.17 The diminished aortic component of the second sound in normotensive patients after a recent infarction or in heart failure, therefore, appears to reflect an impaired rate of isovolumic relaxation. If the aortic valve during diastole can be considered to vibrate analogously to a vibrating membrane, then the driving force productive of valvular vibration is the diastolic pressure gradient.13 Acoustical theory indicates the amplitude of sound generated by a vibrating source in the form of a piston relates directly to the velocity of the vibrating source.18 Any hemodynamic or physiological factors that affect the velocity of the vibrating source would directly affect the amplitude of sound. In the case of a vibrating membrane, which in many ways is similar to the aortic valve, the velocity of deflection of the membrane relates to the rate of change of the diastolic pressure gradient.13 Since the rate of change of the diastolic pressure gradient is affected by negative dp/dt, changes of the rate of isovolumic relaxation will result in changes of the amplitude of the aortic component of the second sound. The diminished aortic sound in aortic stenosis would seem to relate to increased stiffness of the valve."9 Because of the important effect that reduced flexibility of the leaflets may have upon the aortic component of the second sound, particular care was taken to assure that the aortic valve was normal in these patients. Regurgitant valves also are accompanied by a diminished amplitude of the second sound.16 Presumably, in an insufficient valve, the ability of the closed cusps to vibrate is diminished. Therefore, patients with aortic insufficiency were also excluded from this study. Other factors, not yet explored, perhaps may contribute to the second sound. These include the size and shape of the ventricle, the sound absorption of the aortic and left ventricular walls, and the viscosity of the blood.'3 In summary, in patients with poor ventricular performance, the rate of isovolumic relaxation may be compromised. This would cause a reduction of negative dp/dt which in turn causes a reduction of the rate of change of the pressure gradient that develops across the valve during diastole. A diminished second sound, therefore, would result due to the more slowly developing driving pressure which directly affects the characteristics of valvular vibration. References 1. Camille L: Auscultation. In Cardiology, vol 2, Methods, Edited by Luisada AA. New York, McGraw Hill, 1959, pp 3-100 S1 IN ACUTE MYOCARDIAL ISCHEMIA/Clarke et al. 2. Fowler NO: Cardiac Diagnosis. New York, Harper and Row, 1969, p 46 3. Friedberg CK: Diseases of the Heart, ed. 3. Philadelphia, WB Saunders, 1966, p 807 4. Berne RM, Levy MN: Cardiovascular Physiology, ed 2. St. Louis, CV Mosby, 1972, p 77 5. Levine SA, Harvey WP: Clinical Auscultation of the Heart, ed 2. Philadelphia, WB Saunders, 1959, pp 38-40 6. Schwartz ML, Little RC: Physiologic basis for the heart sounds and their clinical significance. N Engl J Med 264: 280, 1961 7. Kusukawa R, Bruce DW, Sakamoto T, MacCanon DM, Luisada AA: Hemodynamic determinants of the amplitude of the second heart sound. J Appl Physiol 21: 938, 1966 8. Stein PD, Sabbah HN, Blick EF: Dependence of the intensity of the aortic closure sound upon the rate of ventricular diastolic relaxation and ventricular performance. (abstr) Am J Cardiol 39: 285, 1977 9. Rushmer RF: Cardiovascular Dynamics, ed 3. Philadelphia, WB Saunders, 1970, pp 301-307 10. Piemme TE, Barnett GO, Dexter L: Relationship of heart sounds to acceleration of blood flow. Circ Res 18: 303, 1966 11. Sabbah HN, Stein PD: An investigation of the theory and mechanism of the origin of the second heart sound. Circ Res 39: 875, 1977 593 12. Sabbah HN, Stein PD: Semilunar valvular vibration in early diastole: Its significance in the origin of the second heart sound. (abstr) Proc 30th Ann Conf Engin Med Biol, Los Angeles, 1977, p 391 13. Blick EF, Sabbah HN, Stein PD: Vibration model of semilunar valvular motion during early diastole: Its relevance to determination of the amplitude of semilunar closure sounds. (abstr) Proc 30th Ann Conf Engin Med Biol, Los Angeles, 1977, p 330 14. Hugenholtz PG, Ellison RC, Urschel CW, Mirsky T, Sonnenblick EH: Myocardial force-velocity relationships in clinical heart disease. Circulation 41: 191, 1970 15. Dodge HT, Baxley WA: Left ventricular volume and mass and their significance in heart disease. Am J Cardiol 23: 528, 1969 16. Sabbah HN, Khaja F, Anbe DT, Stein PD: The aortic closure sound in pure aortic insufficiency. Circulation 56: 859, 1977 17. Stein PD, Barr I, Sabbah HN: Amplitude of the second heart sound, diastolic relaxation, and implications related to the mechanism of the second sound. (abstr) The Physiologist 20: 91, 1977 18. Swenson GW Jr: Principles of Modern Acoustics. Cambridge, Boston Technical Publ Inc, 1965, p 114 19. Stein PD, Sabbah HN: Origin of the second heart sound: Clinical relevance of new observations. Am J Cardiol 41: 108, 1978 Downloaded from http://circ.ahajournals.org/ by guest on June 17, 2017 Spectral Energy of the First Heart Sound in Acute Myocardial Ischemia A Correlation with Electrocardiographic, Hemodynamic, and Wall Motion Abnormalities W. BROMLEY CLARKE, PH.D., STEPHEN M. AUSTIN, M.D., PRAVIN M. SHAH, M.D., PAUL M. GRIFFEN, JAMES T. DOVE, M.D., JUDITH MCCULLOUGH, AND BERNARD F. SCHREINER, M.D. SUMMARY First heart sound (S,) energy spectra in isovolumic systole, hemodynamics, and angiographic left ventricular wall motion (LVWM) at rest and with atrial pacing were compared in 27 patients who underwent diagnostic cardiac catheterization and angiography because of chest pain. Eighteen patients were found to have coronary artery disease (CAD) and nine patients, normal coronary arteries. Eleven of the 18 CAD patients (61%) had a mean reduction in the spectral energy of S, of 6.5 ± 1.4 (SEM) dB below control (-52%), during interruption of ischemic stress of rapid atrial pacing, compared to only one of nine patients without CAD (P < 0.05). Only five CAD patients (28%) had an abnormal rise (_5 mm) in left ventricular end-diastolic pressure (LVEDP) either during or upon interrup- tion of pacing, and six (33%) had ischemic ST-segment depression >0.1 mv in the ECG. Similarly two patients free of CAD (22%) had an abnormal increase in LVEDP, and none had ECG evidence of ischemia. Seventeen CAD patients (94%) had segmental LVWM abnormalities at rest or with interruption of pacing, while three patients with normal coronary arteries (33%) had abnormal angiographic LVWM (P < 0.01). Thus, reduction in S, spectral energy is a common accompaniment of myocardial ischemia. In the present study, it was more frequently observed than abnormalities in either the ECG or LVEDP, but was not as consistently seen as segmental left ventricular wall motion abnormalities. ACUTE MYOCARDIAL ISCHEMIA has been noted in the clinical setting to be associated with reduction in loudness of the first heart sound (S,). This study was designed to determine whether detailed analysis of the first heart sound during ischemic stress of rapid atrial pacing provides a quantitative measure of changes in energy spectra From the Cardiology Unit, Department of Medicine, University of Rochester Medical Center, Rochester, New York, and the Corporate Research and Development Center, General Electric Corporation, Schenectady, New York. Supported in part by grants HL 03996 and HL 05500 from the National Heart and Lung Institute, National Institutes of Health, Bethesda, Maryland. Address for reprints: Pravin M. Shah, M.D., Wadsworth VA Hospital, Cardiology Division, Wilshire & Sawtelle Blvds., Los Angeles, California 90073. Received August 10, 1977; revision accepted October 10, 1977. of S5. Several theories have been proposed to explain the mechanism of generation of the first heart sound (S,).'-' The more recent studies3 9 have supported the acceleration and deceleration theory of Rushmer7 as the most likely physiologic explanation. An echocardiographic study from our laboratory9 has shown that, although the timing of mitral valve closure is a critical factor in determining the intensity of S, the energy of sound vibrations is probably derived from the force of left ventricular contraction during isovolumic systole. Sakamoto and associates5 have reported a nearly linear relationship between the amplitude of S, and peak rate of Exploration of the cause of the low intensity aortic component of the second sound in nonhypotensive patients with poor ventricular performance. P D Stein, H N Sabbah, F Khaja and D T Anbe Downloaded from http://circ.ahajournals.org/ by guest on June 17, 2017 Circulation. 1978;57:590-593 doi: 10.1161/01.CIR.57.3.590 Circulation is published by the American Heart Association, 7272 Greenville Avenue, Dallas, TX 75231 Copyright © 1978 American Heart Association, Inc. All rights reserved. Print ISSN: 0009-7322. Online ISSN: 1524-4539 The online version of this article, along with updated information and services, is located on the World Wide Web at: http://circ.ahajournals.org/content/57/3/590 Permissions: Requests for permissions to reproduce figures, tables, or portions of articles originally published in Circulation can be obtained via RightsLink, a service of the Copyright Clearance Center, not the Editorial Office. Once the online version of the published article for which permission is being requested is located, click Request Permissions in the middle column of the Web page under Services. Further information about this process is available in the Permissions and Rights Question and Answer document. Reprints: Information about reprints can be found online at: http://www.lww.com/reprints Subscriptions: Information about subscribing to Circulation is online at: http://circ.ahajournals.org//subscriptions/