* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download A novel mechanism for oral controlled release of drugs by
Survey
Document related concepts
Transcript
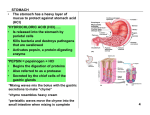
Gastroretentive Dosage Forms Selected Topics in Pharmaceutical Technology Gastroretentive Dosage Forms • Drugs can have poor bioavailability after peroral administration due to slow dissolution, poor permeation and/or degradation in the gastrointestinal (GI) tract. • The poor bioavailability of some of these drugs may be due to their narrow absorption window (NAW) at the upper part of the gastrointestinal tract. • The proximal part of the small intestine exhibits good absorption properties (including high surface area (villi) larger gaps between the tight junctions, and dense active transporters). • Despite the extensive absorption properties of the duodenum and jejunum, the extent of absorption at these sites is limited because the passage through this region is rapid. Gastroretentive Dosage Forms • Enhancing the gastric residence time (GRT) of a NAW drug may significantly improve the net extent of its absorption. Gastroretentive Dosage Forms • Extended release DDS possessing gastric retention properties may be potentially useful as the retention of oral dosage forms in the upper GIT causes prolonged contact time of drug with the GI mucosa, leading to: – Higher bioavailability, and hence therapeutic efficacy – Reduced time intervals for drug administration – Potentially reduced dose size and thus improved patient compliance Gastroretentive Dosage Forms Drug Candidates for Gastric Retention • Gastroretentive DDSs exhibiting controlled drug release are significantly important for drugs which are: – Acting locally in the stomach (e.g. antibiotics against Helicobacter Pylori, antacids and misoprostol) – Absorbed incompletely due to a relatively narrow window of absorption in the GIT, such as cyclosporin, ciprofloxacin, furosemide, L-DOPA and riboflavin. – Unstable in the intestinal or colonic environment such as captopril – Exhibit low solubility at high pH values such as verapamil HCl, diazepam and chlordiazepoxide Gastroretentive Dosage Forms Drug Candidates for Gastric Retention • Gastroretentive DDS, on the other hand, are not suitable for drugs: – That may cause gastric lesions, e.g., non-steroidal antiinflammatory agents – Drug substances that are unstable in the strong acidic environment of the stomach. – In addition, gastroretentive systems do not offer significant advantages over conventional dosage forms for drugs which are absorbed throughout the gastrointestinal tract. Approaches to Gastric Retention • The most important parameters affecting gastric emptying and, hence, the gastric retention time of oral dosage forms include: – 1. Density, size and shape of the device. – 2. Concomitant ingestion of food and its nature, caloric content and frequency of intake. – 3. Simultaneous administration of drugs with impact on gastrointestinal transit time; for example, drugs acting as anticholinergic agents (e.g. atropine, propantheline), opiates (e.g. codeine) and prokinetic agents (e.g. metoclopramide, cisapride). – 4. Biological factors such as gender, posture, age, sleep, body mass index, physical activity and disease states (e.g. diabetes, Crohn's disease). Gastroretentive Dosage Forms • The main approaches that have been examined for gastroretentive drug delivery include: – low density of the GRDF that causes buoyancy above gastric fluid – high density which retains within folds the dosage form (DF) in the body of the stomach that is anatomically lower than the pyloric sphincter – concomitant administration of drugs or excipients which slow the motility of the gastrointestinal tract – bioadhesion to gastric mucosa – swelling to a large size which prevents emptying of the DF through the pyloric sphincter APPROACHES TO ACHIEVE GASTRIC RETENTION Bioadhesive drug delivery systems • It involves the use of bioadhesive polymers that can adhere to the epithelial surface of the GIT. • A bioadhesive can be defined as a substance with the ability to interact with biological materials and is capable of being retained on the biological substrate for a period of time. Bioadhesive drug delivery systems • Bioadhesive polymers are usually macromolecular, hydrophilic gelling substances with numerous hydrogenbond forming groups (carboxyl, hydroxyl, amide and sulfate groups): – polyacrylic acids (Carbapol), sodium carboxymethyl cellulose (CMC), sodium alginate and carrageenan. • Anionic polymers have been found to have better binding capacity than neutral or cationic polymers. Formulation and drug incorporation: Several approaches have been investigated for the incorporation of drug into mucoadhesive polymers onto and/or in cores (maultiparticlute system, such as pellets, granules and microcapsules) 1 Coating: An immediate release core is coated with the mucoadhesive polymer. In this case, the duration of retention is controlled by the dissolution rate of the polymer. Cross-linking can retard this, however, it also reduce the rate of hydration, which negatively affect mucoadhesion. Dose dumping (premature drug release) can result when the coat separate. 2 Matrix type: The drug is dispersed in a matrix of the mucoadhesive polymer. 3 Hybrid: Matrix coated with a polymer. Bioadhesive drug delivery systems • The proposed mechanism of bioadhesion is the formation of hydrogen – and electrostatic bonding at the mucus-polymer boundary. • Rapid hydration in contact with the muco-epithelial surface appears to favor adhesion. Size-increasing drug delivery systems • Another approach to retaining a pharmaceutical dosage form in the stomach is by increasing its size above the diameter of the pylorus . • However, owing to significant inter-individual variations, the cut-off size cannot be determined exactly. • Roughly, the dosage forms should be larger than 13 mm, Size-increasing drug delivery systems • In order to facilitate swallowing, it is highly desirable to design dosage forms with an initially small size that — once in the stomach — significantly increase in size. • The expanded state should be achieved rapidly in order to prevent premature emptying through the pylorus. • Conversely, the systems should also guarantee their clearance from the stomach after predetermined time intervals to avoid accumulation upon multiple administrations. • In addition, the dosage form should have no effect on gastric motility or emptying process. Size-increasing drug delivery systems • Deshpande et al. (Deshpande et al., 1997a; Deshpande et al., 1997b) developed a controlled-release gastric retention system composed of: – a swellable core, which consisted of the drug, chlorphenamine maleate or riboflavin 5′ phosphate, and the expanding agents crosslinked polyvinyl pyrrolidone (PVP), Carbopol 934P and calcium carbonate. – The tablet core was coated with a permeable coating, consisting of blends of Eudragit RL® 30 D and NE 30 D in different ratios. • The tablets swelled to 2- 4 times their original volume, while releasing the drug in a controlled manner. • The optimal ratio of Eudragit® RL 30 D: NE 30 D was found to be 70: 30, which was optimum for sufficient elasticity to withstand the pressure of expansion during the initial swelling phase, and allowing the breakdown of the tablet following release of the drug. Floating drug delivery systems • Drug delivery systems that float immediately upon contact with gastric fluids present promising approaches for increasing the bioavailability of drugs with absorption windows in the upper small intestine. • However, immediate floating can only be achieved if the density of the device is low at the very beginning. • Devices with an initially high density (which decreases with time) first settle down in the stomach and, thus, undergo the risk of premature emptying. • Inherent low density can, for example, be provided by the entrapment of air or by the (additional) incorporation of low-density materials (e.g. fatty substances or oils, or foam powder). Hydrodynamically Balanced System These systems contain one or more hydrocolloids and are made into a single unit along with drug and other additives. When coming in contact with water, the hydrocolloids at the surface of the system swell slowly and facilitate floating by achieving density lower than 1. The coating forms a viscous barrier, and the inner polymer slowly gets hydrated as well, facilitating the controlled drug release by controlling solvent into the tablet penetration and drug diffusion • The polymers used in this system includes hydroxypropylmethylcellulose,hydroxy ethylcellulose, hydroxypropylcellulose, sodium carboxymethylcellulose, agar, carrageenans, and alginic acid. Effervescent matrix This system can be made as tablets, pellets or granules. These buoyant systems utilize matrices prepared with swellable polymers like methocel, polysaccharides like chitosan, effervescent components like sodium bicarbonate, citric acid and tartaric acid. As sodium bicarbonate reacts with HCl in the stomach or incorporated citric acid and or tartaric acid CO2 is released and entrapped in the gelling polymer, which causes rapid floating as the gas has low density High density drug delivery systems • These devices use their weight as a retention mechanism. • When the density of the system is larger than that of the gastric juice (~1.004 g/cm³), the device settles down to the bottom of the stomach, and remains located below the pylorus. • This could be accomplished by including a heavy inert material such as zinc oxide, titanium dioxide, iron powder or barium sulphate into the drug containing core pellets or coating drug containing pellets with it. • These materials increase density by up to 1.5–2.4 g/cm3