* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download Chemistry of Life
Survey
Document related concepts
Cholesterol wikipedia , lookup
High-density lipoprotein wikipedia , lookup
Epoxyeicosatrienoic acid wikipedia , lookup
Ethanol-induced non-lamellar phases in phospholipids wikipedia , lookup
15-Hydroxyeicosatetraenoic acid wikipedia , lookup
Phospholipid-derived fatty acids wikipedia , lookup
Transcript
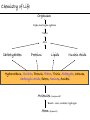
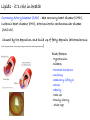
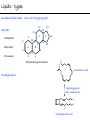
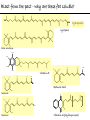
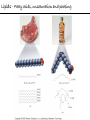
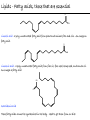
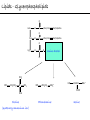
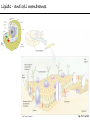
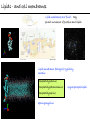
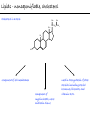
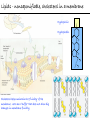
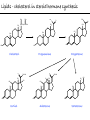
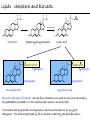
Chemistry of Life Organism Organ and organ systems Tissue Cells Carbohydrates Proteins Lipids Nucleic Acids Hydrocarbons, Alcohols, Phenols, Ethers, Thiols, Aldehydes, Ketones, Carboxylic Acids, Esters, Amines, Amides Molecules (compounds) Bonds - ionic, covalent, hydrogen Atom (elements) Chemistry of Life - Goals At the end of this chapter you should: • be able to identify the different classes of lipids. • know the difference between saturated and unsaturated fatty acids. • be able to describe the effect of chain length and unsaturation on the melting point of fatty acids. • know the difference between fats and oils and be able to describe how the fatty acid composition determines the physical properties of fats and oils. • be familiar with the hydrolysis and saponification reactions of triglycerides. • be able to describe the properties of glycerophospholipids and their role in the structure of cell membranes. • be able to describe the role of cholesterol in human health. • be familiar with the role of lipoproteins in transporting lipids and know the difference between LDL and HDL particles. • be familiar with the importance of lipids in human health. Lipids - it’s role in health Coronary Artery Disease (CAD) - aka coronary heart disease (CHD), ischemic heart disease (IHD), arteriosclerotic cardiovascular disease (ASCVD). Caused by the deposition and build up of fatty deposits (atherosclerosis: from the greek ‘athera’ meaning porridge and sclerosis meaning hardening) Risk factors: - Hypertension - Diabetes - Elevated cholesterol - smoking - Sedentary lifestyle - Stress - Obesity - Male sex - Family history - Older age Lipids - it’s role in health - What are triglycerides? - What is HDL? - What is LDL? - How are there molecules made? what is their chemistry? Lipids (from the greek ‘lipos’ meaning fat or lard) - types Lipids are a class of molecules that are insoluble in water but are soluble in non-polar organic solvents. Lipids Saponifiable lipids (can be hydrolyzed) non-Saponifiable lipids (can NOT be hydrolyzed) - Waxes - Steroids - Triglycerides - Prostaglandins - Glycerophospholipids - Sphingolipids - Glycosphingolipids Remember the esters? Lipids - types Saponifiable lipids - (can be hydrolyzed) Remember the esters? Fatty acid long chain alcohol Waxes G l y c e r o l G l y c e r o l Fatty acid Fatty acid Fatty acid Triglycerides S p h i n g o s i n e Fatty acid PO4 Amino alcohol Sphingolipids Fatty acid Fatty acid PO4 Amino alcohol Glycerophospholipids S p h i n g o s i n e Fatty acid Sugar Glycosphingolipids Lipids - types nonSaponifiable lipids - (can NOT be hydrolyzed) 12 Steroids Cholesterol Bile salts Hormones 11 2 3 1 10 9 A 4 B 5 C 13 17 D 16 14 15 8 7 6 Steroid Ring Structure OH Arachidonic acid O Prostaglandins Cyclooxygenase (COx-1 and COx-2) O COOH O OH (prostaglandin H2) Lipids - saponifiable, fatty acids in common Saponifiable lipids - (can be hydrolyzed) Remember the esters? Fatty acid long chain alcohol Waxes G l y c e r o l G l y c e r o l Fatty acid Fatty acid Fatty acid Triglycerides S p h i n g o s i n e Fatty acid PO4 Amino alcohol Sphingolipids Fatty acid Fatty acid PO4 Amino alcohol Glycerophospholipids S p h i n g o s i n e Fatty acid Sugar Glycosphingolipids Lipids - Fatty acids O OH Palmitic acid (hexadecanoic acid) - a saturated fatty acid found in palm oil, butter, cheese, milk. O OH Stearic acid (octadecanoic acid) - a saturated fatty acid from plant and animal fats and oils. O OH Linoleic acid - a poly unsaturated fatty acid from plant and animal fats and oils. An omega-6 fatty acid. O OH Linolenic acid - a poly unsaturated fatty acid from fish oil, flax seed, hemp seed, and canola oil. An omega-3 fatty acid. Lipids - Fatty acids, solubility Tail Water soluble polar hydrophilic (water liking) O OH Water insoluble Non polar hydrophobic (water fearing) Head group The hydrophobic tail is responsible for the fatty or oily characteristics of fatty acid containing lipids Lipids - Fatty acids, solubility and micells The hydrophobic tails associate into the structure. The hydrophilic head group points towards the water. Micelles Held together by weak dispersion forces. Blast from the past - why are these fat soluble? Hydrophobic Lycopene beta-carotene CH3 HO CH3 O H3C CH3 CH3 CH3 CH3 H3C H3C CH3 CH3 CH3 OH Vitamin E CH3 CH3 CH3 CH3 O H CH3 O Retinoic acid CH3 Retinal H3C O CH3 CH3 CH3 * 3 OH Retinol CH3 O Vitamin K(phylloquinone) Lipids - Fatty acids, they are not flat O OH Palmitic acid (hexadecanoic acid) - a saturated fatty acid found in palm oil, butter, cheese, milk. O OH Stearic acid (octadecanoic acid) - a saturated fatty acid from plant and animal fats and oils. O OH Linoleic acid - a poly unsaturated fatty acid from plant and animal fats and oils. An omega-6 fatty acid. O OH Linolenic acid - a poly unsaturated fatty acid from fish oil, flax seed, hemp seed, and canola oil. An omega-3 fatty acid. Lipids - Fatty acids, three dimensional shape Lipids - Fatty acids, three dimensional shape Lipids - Fatty acids, some examples and their properties Lipids - Fatty acids, unsaturation and packing Lipids - Fatty acids, fish oils and the heart O OH docosahexaenoic acid (DHA) O OH eicosapentaenoic acid (EPA) Polyunsaturated fatty acids (PUFAs), learn more at the AHA Lipids - Fatty acids, trans fatty acids and health The process that is used to convert polyunsaturated vegetable oils (liquids) to partially hydrogenated solid fats to be used in margarine and shortening produces trans fats. H H C H3C CH2 CH2 C H C C H3C CH2 H C CH3 H C CH2 CH2 H partial hydrogenation cis-2,5-octadiene Learn more qbout Trans Fatty acids at: (1). The FDA (2). About Trans Fatty acids in butter and margarine at the AHA (2). About Trans Fatty acids and heart disease at the AHA Fatty acids are thought to (1). Increase the levels of LDL (Low Density Lipoproteins) (2). Reduces the levels of HDL (high Density Lipoproteins) trans-3-octene CH2 CH3 Lipids - Fatty acids, those that are essential O OH Linoleic acid - a poly unsaturated fatty acid from plant and animal fats and oils. An omega-6 fatty acid. O OH Linolenic acid - a poly unsaturated fatty acid from fish oil, flax seed, hemp seed, and canola oil. An omega-3 fatty acid. OH O Arachidonic acid These fatty acids cannot be synthesized in the body. Need to get them from our diet. Lipids - Waxes O CH3(CH2)18 C O (CH2)19CH3 Jojoba wax O CH3(CH2)14 C O (CH2)29CH3 Bees wax O CH3(CH2)24 C Carnauba wax O (CH2)29CH3 Lipids - Fats and Oils Fats and Oils - are esters of glycerol. Also known as triglycerols or triglycerides. Fatty acids in the body are stored as triglycerides. Triglycerides that are (usually) liquids at room temperature are oils. Triglycerides that are solids at room temperature are fats. G l y c e r o l O Fatty acid CH2 O C O (CH2)16CH3 CH O C O (CH2)16CH3 CH2 O C (CH2)16CH3 Fatty acid Fatty acid Triglycerides Glyceryl tristearate Lipids - Fats and Oils, triglycerides O CH2 OH CH OH HO (CH2)16CH3 O + HO CH2 C C (CH2)16CH3 OH O HO Glycerol C (CH2)16CH3 stearic acid Three of them. One for each alcohol group. Esterification Ester group O CH2 O C O (CH2)16CH3 CH O C O (CH2)16CH3 CH2 O C (CH2)16CH3 Glyceryltristearate Lipids - Fats and Oils, triglycerides O Alcohol Part In triglycerides always Glycerol CH2 O C O (CH2)16CH3 CH O C O (CH2)16CH3 CH2 O C (CH2)16CH3 Carboxylic acid part Three fatty acids Glyceryltristearate In Glyceryltristearate all three fatty acids are the same. But they do not have to be... O CH2 O C O (CH2)16CH3 from stearic acid CH O C O (CH2)14CH3 from Palmitic acid Saturated fats CH2 O C (CH2)12CH3 from Myristic acid Unhealthy In Glyceryltristearate all three fatty acids are saturated. But they do not have to be... O CH2 O C O (CH2)16CH3 CH O C O (CH2)7CH CH2 O C (CH2)12CH3 from stearic acid CH(CH2)5CH3 from Palmitoleic acid from Myristic acid unSaturated fats Lipids - Fats and Oils, triglycerides Triglycerides from animal sources are usually solids at room temperature - they are called Fats. There are exceptions the most notable being the triglycerides from fish. Triglycerides from animal plant sources are usually liquids at room temperature - they are called oils. Think back to the melting points of different fatty acids. Can you predict what types of fatty acids are prevalent in animal vs. plant triglycerides? Lipids - Fats and Oils, triglycerides Physical properties O CH2 O C O (CH2)16CH3 CH O C O (CH2)7CH CH2 O C (CH2)12CH3 hydrophobic tail CH(CH2)5CH3 Ester group not as hydrophillic as the fatty acid carboxyl group. Overall more hydrophobic than fatty acids. Do not form micells. Compare To hydrophilic Head group O OH hydrophobic Tail Lipids - Fats and Oils, triglycerides Chemical properties What groups do you think will determine the chemical properties of triglycerides? Ester group O CH2 O C O (CH2)16CH3 CH O C O (CH2)7CH CH2 O C (CH2)12CH3 Alkene group CH(CH2)5CH3 The ester and alkene functional groups will determine the chemical properties of triglycerides. Lipids - Fats and Oils, triglycerides Chemical properties, Hydrolysis O O Heat/H+ CH3 C O CH3 + H2O CH3 Ester C OH carboxylic acid CH2 O C O (CH2)16CH3 CH O C O (CH2)7CH O C OH Alcohol O O CH2 + CH3 (CH2)12CH3 Triglycerol - the ester H2O/ acid CH(CH2)5CH3 CH2 OH CH OH CH2 HO (CH2)16CH3 O + HO C (CH2)7CH O OH Triglycerol - the alcohol C HO C (CH2)12CH3 Fatty acids - the carboxylic acids CH(CH2)5CH3 Lipids - Fats and Oils, triglycerides Chemical properties, saponification O O CH3 C O CH3 + NaOH CH3 Ester C carboxylate ion CH CH2 O O O C O (CH2)16CH3 C O (CH2)7CH C OH Alcohol O O CH2 + CH3 O- Na+ CH2 OH CH OH +Na-O (CH2)12CH3 Triglycerol - the ester CH2 OH Triglycerol - the alcohol (CH2)16CH3 O NaOH CH(CH2)5CH3 C + +Na-O C (CH2)7CH CH(CH2)5CH3 O +Na-O C (CH2)12CH3 Salts of the Fatty acids - the salts of the carboxylic acids These are soaps Coconut shampoo - which oil does this come from? Lipids - Fats and Oils, triglycerides Chemical properties, Hydrogenation O O CH2 O C O (CH2)7CH CH(CH2)5CH3 CH O C O (CH2)7CH CH(CH2)5CH3 CH2 O C (CH2)12CH3 H2/Ni CH2 O C O (CH2)14CH3 CH O C O (CH2)7CH CH2 O C (CH2)12CH3 Partial hydrogenation Used to convert vegetable oils to produce spreadable fats (like margarine). Causes the production of trans fats. Learn more qbout Trans Fats at: (1). The FDA (2). About Trans Fatty acids in butter and margarine at the AHA (2). About Trans Fatty acids and heart disease at the AHA Trans Fats are thought to (1). Increase the levels of LDL (Low Density Lipoproteins) (2). Reduces the levels of HDL (high Density Lipoproteins) CH(CH2)5CH3 Trans Fats: Coming in 2006, to a label near you... Trans Fats: Coming in 2006, to a label near you... Lipids - Fats and Oils, the hunt for new fats The glycerol molecule in triglycerides had three alcohol groups to form esters. What other molecules have we seen with multiple alcohol groups? O CH2 O C = fatty acid O O Acid Fatty Fatty Acid O O Fatty Acid O Acid Fatty O CH2 O O O Acid Fatty Fatty Acid Fatty Acid O Olestra - a fat substitute made by combining sucrose with fatty acids no enzymes to break it down, large size prevents absorption. Contributes no calories. May lead to lipid soluble vitamin (A, D, E and K) deficiency. Lipids - Fats and Oils, triglycerides - What are triglycerides? Are triesters of glycerol and fatty acids. - those that are liquid at room temperature are oils. - those that are solids at room temperature are Fats. - triglycerides have chemical properties of esters and alkenes. - triglycerides are the major source of energy storage of animals. - triglycerides with a high content of saturated fatty acids or with trans fatty acids are unhealthy. Lipids - types Saponifiable lipids - (can be hydrolyzed) Remember the esters? Fatty acid long chain alcohol Waxes G l y c e r o l G l y c e r o l Fatty acid Fatty acid Fatty acid Triglycerides S p h i n g o s i n e Fatty acid PO4 Amino alcohol Sphingolipids Fatty acid Fatty acid PO4 Amino alcohol Glycerophospholipids S p h i n g o s i n e Fatty acid Sugar Glycosphingolipids Lipids - Glycerophospholipids G l y c e r o l Fatty acid Fatty acid PO4 Compare To Amino alcohol Glycerophospholipids G l y c e r o l Fatty acid Fatty acid Fatty acid Triglycerides In glycerophospholipids one of the hydroxyl groups in glycerol is bonded to a phosphate (PO4) via an ester link. The phosphate is in turn linked to another alcohol (usually an amino alcohol), via another ester link. These lipids are also known as phospholipids. Lipids - Glycerophospholipids Fatty Acids glycerol G l y c e r o l Fatty acid H2C O O C (CH2)7CH CH(CH2)5CH3 (CH2)7CH CH(CH2)5CH3 O CH Fatty acid O C O PO4 H2C Amino alcohol O P CH3 O O- phosphate O H2C O C (CH2)7CH CH(CH2)5CH3 (CH2)7CH CH(CH2)5CH3 O CH O C O H2C O P O- CH3 O CH2CH2 N+ CH3 CH3 Ester links (phosphodiester) CH2CH2 N+ CH3 CH3 amino alcohol (choline) Lipids - Glycerophospholipids O H2C O C (CH2)7CH CH(CH2)5CH3 (CH2)7CH CH(CH2)5CH3 O CH O C O H2C O P CH3 O CH2CH2 alcohol N+ CH3 amino O- CH3 CH3 HO CH2CH2 N+ CH3 HO CH2CH2 NH3+ HO CH2CH COO- CH3 Choline (quatenary ammonium ion) Ethanolamine Serine NH3+ Lipids - Glycerophospholipids O H2C O C (CH2)7CH CH(CH2)5CH3 (CH2)7CH CH(CH2)5CH3 O CH O C O H2C O P CH3 O CH2CH2 O- N+ CH3 CH3 phosphatidyl choline A typical phosphoglyceride (glycerophospholipid). Also called lecithin. All phosphoglycerides that contain a choline are called lecithins. The fatty acids can be the same or differet. Can be saturated or unsaturated or can be a mixture. Lipids - Glycerophospholipids O Physical properties H2C O C (CH2)7CH CH(CH2)5CH3 (CH2)7CH CH(CH2)5CH3 hydrophobic tails O CH O C O H2C O P CH3 O CH2CH2 O- N+ CH3 hydrophillic head group CH3 The negatively charged phosphate and the positively charges ammonium group make the head group hydrophillic. Glycerophospholipids form micells. O CH2 O C O (CH2)16CH3 CH O C O (CH2)7CH CH2 O C (CH2)12CH3 hydrophobic tail CH(CH2)5CH3 hydrophilic Head group O OH hydrophobic Tail Lipids - Glycerophospholipids The 3D structure Palmitoyl Oleoyl phosphatidyl choline Lipids - and cell membranes Lipids - and cell membranes Lipid membranes are ‘fluid’ - they permit movement of proteins and lipids. Lipid membranes (Bilayers) typically contain: Phosphatidylcholine Phosphatidylethanolamine Phosphatidylserine Sphingomyelins Glycerophospholipids Lipids (from the greek ‘lipos’ meaning fat or lard) - types Lipids are a class of molecules that are insoluble in water but are soluble in non-polar organic solvents. Lipids Saponifiable lipids (can be hydrolyzed) non-Saponifiable lipids (can NOT be hydrolyzed) - Waxes - Steroids - Triglycerides - Prostaglandins - Glycerophospholipids - Sphingolipids - Glycosphingolipids Remember the esters? Lipids - nonsaponifiable - Steroids - they have the same distinguishing feature of the saponifiable lipids, i.e. they do not dissolve in water. BUT their structures are completely different. 12 11 2 3 1 10 9 A 4 B 5 C 13 17 D 16 14 15 8 7 6 Steroid Ring Structure The most important and most abundant steroid is cholesterol. Lipids - nonsaponifiable, cholesterol Cholesterol is a sterol CH3 CH3 CH3 CH(CH2)3CHCH3 CH3 HO component of cell membranes component of myelin sheath, nerve and brain tissue used in the synthesis of other steroids including steroid hormones, bile salts, and vitamin D etc. Lipids - nonsaponifiable, cholesterol in a membrane HO Hydrophilic Hydrophobic 3 CH 3 CH C 3 CH 2 )3 C H( CH 3 CH 3 HC H Cholesterol helps maintain the ‘fluidity’ of the membrane. Acts as a ‘buffer’ that does not allow big changes in membrane fluidity. Lipids - cholesterol in steroid hormone synthesis CH3 CH3 CH3 CH3 CH(CH2)3CHCH3 CH3 O O CH3 CH3 CH3 CH3 CH3 O HO HO Cholesterol O Pregnenolone O CH2OH CH3 O OH HO CH3 CH CH3 O Cortisol CH2OH HO CH3 O Progesterone CH3 O aldosterone testosterone OH The steroid hormones Progesterone Prepares uterus for fertilized egg testosterone Development of male organs; male sexual characteristics; sperm formation Cortisol (a glucocorticoid) Stimulated glycogen synthesis from amino acids and increased blood glucose levels. Inhibits allergic and inflammatory responses. aldosterone (a mineralocorticoid) Helps maintain Na and K balance in tissues; water retention. Lipids - cholesterol and bile salts CH3 CH3 CH3 CH3 CH(CH2)3CHCH3 CH3 Cholesterol OH CH3 CH3 O CHCH2CH2 C NH CH2 CH2 Taurocholic acid Hydrophobic O CHCH2CH2 C O- OH Cholic acid OH SO3- CH3 Hydrophilic OH CH3 CH3 HO OH 7alpha-hydroxycholesterol CH3 HO CH3 Many steps HO HO OH CH(CH2)3CHCH3 CH3 CH3 CH3 CH3 O CHCH2CH2 C NH CH2 C O- Hydrophilic CH3 HO O OH Hydrophobic Glycocholic acid Bile satls (the salts of the acid) - derived from cholesterol; are made in the liver and stored in the gallbladder; secreated in to the intestine after meals to solubilize fats. The contain both hydrophobic and hydrophilic parts and therefore act as very good detergents. The micelles help break up fat in the diet so that they can be broken down. Lipids - cholesterol, the dark side Cholesterol is a major constituent of atherosclerotic plaques. Lipids - cholesterol, a summary - What is cholesterol? - cholesterol is a lipid that is a steroid, a compound that contains a steroid nucleus. - cholesterol is a sterol because it contains a OH group. - cholesterol is a sterol because it contains a OH group. - cholesterol is a major constituent of cellular membranes and helps membrane maintain its rigidity. - cholesterol is needed for the synthesis of other important steroid hormones like progesterone and testosterone, and also for the synthesis of bile salts. - cholesterol is a major component of atherosclerotic plaques and therefore plays an important role in heart disease. Lipids - transporting If triglycerides (the major storage molecule of fatty acids) and cholesterol are mainly hydrophobic (non-soluble in water) then how are they transported in the blood which is mainly water? Use lipoprotein particles. transports lipids from intestine to cells for use as energy and for storage. transports lipids from liver to cells for use as energy and for storage. transports lipids to cells to be used in cell membranes, synthesis of steroids. Excess LDL causes cholesterol to deposit in plaques. transports excess lipids from cells to liver to be converted to bile salts and eliminated. Lipids - transporting - What are LDLs and HDLs? - Low density lipoproteins (LDLs) and High density lipoproteins (HDLs) are lipid/protein particles that are responsible for the transport of lipids in the blood. - LDLs are bad cholesterol, because the cholesterol within the LDL can be deposited in plaques. - HDLs are good cholesterol, because they help remove excess cholesterol to the liver to be eliminated. Lipids - nonsaponifiable, prostaglandins Why are they nonsaponifiable? OH Arachidonic acid O Cyclooxygenase (COx-1 and COx-2) ~ 20 others O COOH O OH (prostaglandin H2) - involved in almost every phase of the reproduction. - involved in blood clotting. - involved in inflammation and pain. Lipids (from the greek ‘lipos’ meaning fat or lard) - types Lipids are a class of molecules that are insoluble in water but are soluble in non-polar organic solvents. Lipids Saponifiable lipids (can be hydrolyzed) non-Saponifiable lipids (can NOT be hydrolyzed) - Waxes - Steroids - Triglycerides - Prostaglandins - Glycerophospholipids - Sphingolipids - Glycosphingolipids Remember the esters?