* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download Document
Remote ischemic conditioning wikipedia , lookup
Rheumatic fever wikipedia , lookup
Cardiothoracic surgery wikipedia , lookup
Cardiac contractility modulation wikipedia , lookup
Electrocardiography wikipedia , lookup
Heart failure wikipedia , lookup
Coronary artery disease wikipedia , lookup
Jatene procedure wikipedia , lookup
Management of acute coronary syndrome wikipedia , lookup
Cardiac surgery wikipedia , lookup
Lutembacher's syndrome wikipedia , lookup
Quantium Medical Cardiac Output wikipedia , lookup
Mitral insufficiency wikipedia , lookup
Ventricular fibrillation wikipedia , lookup
Heart arrhythmia wikipedia , lookup
Arrhythmogenic right ventricular dysplasia wikipedia , lookup
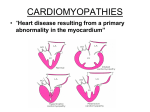
Cardiovascular System Lecture 3 Cardiomyopathy, Pericardial disease & Cardiac Tumors Dr. Samir Al Bashir Cardiomyopathies • Cardiomyopathies have been classified into three forms: – Dilated Cardiomyopathy – Hypertrophic Cardiomyopathy – Restrictive Cardiomyopathy Dilated Cardiomyopathy (DCM) • DCM is the most common form, accounting for about 90% of cases. • Characterized by progressive cardiac hypertrophy, dilation and contractile (systolic) dysfunction (ineffective contraction, patients may have an ejection fraction of less than 25%). • Most common between ages of (20-50 years), men > women. Dilated Cardiomyopathy (DCM) • End stage by time of Dx. & no specific pathologic features at autopsy in majority of cases. • Five general pathways: – Inherited genetic abnormalities (20% to 50% ). – Viral myocarditis: presence of nucleic acids of coxsackievirus B and other enteroviruses. – Toxic chemicals: Alcohol abuse, Cobalt and Chemotherapeutic agents (Doxorubicin). – Pregnancy: occurs late in pregnancy or several weeks post partum (peripartum cardiomyopathy) . – Iron overload: DCM most common manifestation DCM: Genetic and familial conditions • Mutations in cytoskeletal protein genes such as: – Dystrophin gene on chromosome X (Becker and Duchenne muscular dystrophy). – Genes encoding desmin, merosin, and dystrophinassociated proteins termed sarcoglycans. • Abnormalities in certain mitochondrial enzymes. • Mutations in certain sarcomere protein genes (e.g., βmyosin and cardiac troponin T). DCM: Pathology • The heart is enlarged and flabby, weighs 900 g (2-3X normal), there is dilation of all chambers. • Dilation and poor contractile function cause stasis of blood → development of fragile mural thrombi and subsequent emboli. Microscopic features are non-specific • Myocyte hypertrophy • Interstitial fibrosis, wavy fiber change • Iron accumulation with iron overload • Scanty mononuclear infiltrate (sometimes) DCM: Clinical Features • Most cases arise sporadically (except familial cases). • Patients develop progressive congestive heart failure. • Prognosis is very poor (except peripartum DCM): – 50% die within 2yr, 75% die within 5yr due to (CHF, embolic complications or ventricular arrhythmias). • Cardiac transplantation is the only mode of therapy DCM: Four-chamber dilation and hypertrophy. DCM: Histology demonstrating variable myocyte hypertrophy and interstitial fibrosis Hypertrophic Cardiomyopathy (HCM) Asymmetric septal hypertrophy or Idiopathic hypertrophic subaortic stenosis It is characterized by: • Myocardial hypertrophy which causes powerful contractions that rapidly expel blood from the ventricular cavities. • Impaired diastolic filling because of the stiff, thick wall of the ventricle. • Intermittent ventricular outflow obstruction (in many cases) . So the basic problem is an inability to fill a hypertrophic left ventricle during diastole. Ejection is forceful but ineffective because the amount of blood in the left ventricle is greatly reduced. Hypertrophic Cardiomyopathy (HCM) Asymmetric septal hypertrophy or Idiopathic hypertrophic subaortic stenosis Pathogenesis: • In 50% of cases, HCM inherited as an autosomal dominant trait – Mutations in genes encoding sarcomeric contractile proteins (70-80%): • -myosin heavy chain (commonest ) • Troponin T, myosin binding protein C • Allelic heterogeneity HCM: Pathology • Hypertrophy of LV and interventricular septum “IVS”, without dilation (the left atrium may be dilated). (800 gm+) • IVS is thicker than the free (lateral) wall of the left ventricle, and is most evident in subaortic region. • Asymmetric septal hypertrophy is often associated with functional ventricular outflow obstruction during systole (25%) which is caused by abnormal anterior motion of the mitral valve leaflet during systole. • This motion lead to recurrent, forceful contact between the septum and the anterior mitral leaflet causing thickening of the anterior mitral leaflet and adjacent septal endocardium. HCM: Pathology Microscopically • Irregular arrangement of hypertrophied abnormally branching myocytes • Myocardial fibrosis (late stage) HCM: Clinical • • • • • • • Exertional dyspnea Harsh systolic ejection murmur Anginal pain because of myocardial ischemia Atrial fibrillation with thrombus formation Ventricular arrhythmias and sudden death Increased risk of infective endocarditis Later, progressive myocardial fibrosis may cause congestive heart failure. Prognosis: varies with the genetic defect, so molecular diagnosis is useful HCM: There is asymmetric hypertrophy of the septal muscle, and the left atrium is enlarged. HCM: disarray, extreme hypertrophy, and peculiar branching of myocytes as well as interstitial fibrosis Restrictive Cardiomyopathy (RCM) Characterized by primary decrease in ventricular compliance, resulting in impaired ventricular filling during diastole. • It is the least common type of Cardiomyopathy. • The problem is a stiff and inelastic ventricle that can be filled only with great effort. • Myocardial contractility, although often normal early in the course of the disease, usually declines, causing congestive heart failure in later stages. • Symptoms: fatigue, exertional dyspnea, chest pain, and arrhythmias RCM Causes: • Endomyocardial fibrosis (the most common cause) accounts for up to 10% of cases of childhood heart disease in tropical areas. • Eosinophilic endomyocardial fibrosis (Löffler syndrome), is rare. • Genetic factors are not clearly defined, but may account for some cases (desmin mutations) • Additional important causes: – Amyloidosis–systemic or senile cardiac (transthyretin) – Sarcoidosis – Radiation injury to the heart. – Endocardial fibroelastosis RCM: Pathology • Tropical endomyocardial fibrosis and Löffler syndrome: – The atria are dilated, and the ventricles of normal size – The endocardium is thick and solid (left ventricle). – Microscopically: dense fibrosis in endo & myocardium. • Eosinophilic endomyocardial fibrosis: – infiltration by eosinophils (early stages). • Endocardial fibroelastosis (uncommon): – Occurs mostly in children < 2 years of age, – Abundant fibroelastic tissue in the endocardium revealing porcelain-like appearance). Pericardial Effusions Accumulation of fluid in the pericardial space, fluid nature varies with the cause. Major types and their causes are: Serous: congestive heart failure, hypoalbuminemia Serosanguineous: chest trauma, malignancy Chylous: mediastinal lymphatic obstruction Fibrinous / Serofibrinous: RF, connective tissue diseases (SLE), MI and post-MI, trauma & uremia Blood (Hemopericardium): ruptured aortic aneurysms, ruptured myocardial infarcts, penetrating traumatic injury to the heart. Pericarditis: Pericardial Inflammation Pericarditis is usually secondary to: Myocardial infarction, cardiac surgery, or radiation to the mediastinum. Associated with systemic disorders, mostly with uremia Rheumatic fever, systemic lupus erythematosus (SLE), and metastatic malignancies (bloody effusions). Primary Pericarditis is uncommon, mostly of viral origin and sometimes may be caused by other organisms (pyogenic bacteria, mycobacteria and fungi). Pericarditis: Morphology • In uremia, and acute rheumatic fever: the exudate is fibrinous and reveals a shaggy irregular pericardial surface (bread and butter pericarditis). • Viral pericarditis fibrinous exudate. • Acute bacterial pericarditis fibrinopurulent exudate. • Tuberculosis caseous materials. • Pericardial metastases shaggy fibrinous exudate and a bloody effusion. • Chronic Pericarditis: Ranges from slight adhesions to dense fibrotic scars that encases the heart so it cannot expand normally during diastole, a condition called constrictive pericarditis. Pericarditis: Clinical Atypical chest pain (worse on reclining), and harsh friction rub. Significant exudate signs and symptoms of cardiac tamponade (weak distant heart sounds, distended neck veins, declining cardiac output, and shock). Chronic constrictive pericarditis venous distension and low cardiac output. Pericarditis may Cause immediate hemodynamic complications if a significant effusion is present Resolve without significant sequelae Progress to a chronic fibrosing process. Fibrinous Pericarditis Hemorrhagic Pericarditis Cardiac Tumors • Primary heart tumors are rare. • Metastatic neoplasms are seen in up to 10% of patients dying of disseminated cancer, mostly involving pericardium. • The most common primary neoplasms that metastasize to the heart are: – Carcinomas of the lung and breast, – Malignant melanomas, – Lymphomas and leukemias. Metastases may reach the heart via lymphatic, venous, or arterial channels. Metastatic tumor masses involving the myocardium and endocardium. Cardiac tumors Primary tumors include: • Myxoma: benign tumor, occurs mainly in atria, left atrium in about 80% of the cases. They appear as sessile or pedunculated gelatinous mass covered by endothelium. The commonest 1ry adult heart tumor. – Microscopically: multinucleated stellate (Starshaped) cells, edema and mucoid stroma. Cardiac tumors • Rhabdomyoma – Common (infancy and children) – Associated with tuberous sclerosis (neurocutaneous syndrome) – Grossly: myocardial masses project into the ventricular lumen (solitary or multifocal). – Microscopically: eosinophilic polygonal cells (contain large glycogen granules in their cytoplasm). • Lipoma, and Papillary Elastofibromas, • Sarcomas: Angiosarcomas, and Rhabdomyosarcomas. Rhabdomyoma of the left ventricle