* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download Beta-blockers Versus ACE Inhibitors in Heart Failure
Coronary artery disease wikipedia , lookup
Heart failure wikipedia , lookup
Remote ischemic conditioning wikipedia , lookup
Arrhythmogenic right ventricular dysplasia wikipedia , lookup
Cardiac surgery wikipedia , lookup
Management of acute coronary syndrome wikipedia , lookup
Cardiac contractility modulation wikipedia , lookup
Myocardial infarction wikipedia , lookup
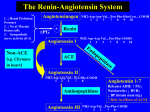
Beta-blockers Versus ACE Inhibitors in Heart Failure with Reduced Ejection Fraction: Which Should be Utilized First? By Genevieve Hale, Pharm.D., BCPS, PGY2 cardiology resident, VA Ann Arbor Healthcare System; and Lindsey Greiner, Pharm.D., PGY1 pharmacy practice resident, VA Ann Arbor Healthcare System Target Audience This continuing education activity was designed specifically for pharmacists. Disclosure Statement The authors do not have any conflicts of interest, or financial relationships with a commercial interest, related to the activity. Learning Objectives At the end of this activity, participants should be able to: define heart failure and distinguish between the different types of heart failure. identify risk factors and causes of heart failure. explain the pathophysiology of heart failure and role of angiotensin-converting enzyme (ACE) inhibitors and beta-blockers in heart failure physiology. explain the landmark trials involving ACE inhibitor and beta-blocker therapy in heart failure. recommend whether ACE inhibitors or beta blockers should be utilized first in heart failure treatment. Introduction Heart failure (HF) is a progressive disease that creates a large burden within the health care system. In Americans aged 40 years and older, the lifetime risk of developing HF is 20 percent.1 More than 650,000 new cases of HF are diagnosed each year.1 Heart failure is associated with excessive hospital admissions, being the primary diagnosis in more than 1 million hospitalizations annually.1 In addition, approximately 50 percent of patients will die within five years of HF diagnosis.1 HF is defined as a complex clinical syndrome that results from any structural or functional impairment of ventricular filing or ejection of blood.1,2 This general term is further classified into types of dysfunction based on left ventricular ejection fraction (LVEF), including HF with reduced ejection fraction (HFrEF), or HF with preserved ejection fraction (HFpEF). An LVEF of less than 40 percent signifies HFrEF, whereas an individual with an LVEF of greater than 50 percent has HFpEF. These are usually accompanied by varying degrees of systolic and diastolic dysfunction. Patients with HFrEF may exhibit systolic and diastolic dysfunction, but those with HFpEF are less likely to have a reduction in systolic function.1-3 A unique aspect of this disease state is the absence of definitive diagnostic testing; therefore, diagnosis is based on clinical judgment. 1-3 According to the European Society of Cardiology, the criteria for a diagnosis of HFrEF requires symptoms and signs of typical HF with a reduced LVEF. The diagnosis of HFpEF requires symptoms and signs of typical HF, normal or only mildly reduced LVEF and LV that is not dilated, and relevant structural heart disease (i.e., LV hypertrophy and/or left atrium enlargement), and/or diastolic dysfunction. 2 The most common symptoms and recognizable signs of HF are edema, dyspnea and fatigue with elevated jugular venous pressure, pulmonary crackles, and displaced apex beats.2 Keeping these symptoms in mind, HF is further classified by functional class and progression of structural disease (see Table 1).1,2 Unfortunately, there are a wide range of underlying pathologies that play a role in the development of HF. First-line agents in the management of HFrEF include angiotensin-converting enzyme (ACE) inhibitors and beta-blockers (BB).1,2 These agents have demonstrated a reduction in mortality and hospitalizations among HF patients. As ACE inhibitors and BB demonstrate clinical benefits in multiple comorbid conditions, comorbidities and patient-specific factors should be considered when determining initial therapy for the management of HF. This article aims to provide an overview of HF and the utilization of ACE inhibitor and BB therapy. Table 1. Stages and Functional Classifications of HF 1 ACCF/AHA Stages of HF A At high risk for HF but without structural heart disease of symptoms of HF B Structural heart disease but without signs or symptoms of HF C Structural heart disease with prior or current symptoms of HF D Refractory HF requiring specialized interventions NYHA Functional Classifications I No limitation of physical activity. Ordinary physical activity does not cause symptoms of HF. II Slight limitation of physical activity. Comfortable at rest, but ordinary physical activity results in symptoms of HF. III Marked limitation of physical activity. Comfortable at rest, but less than ordinary activity causes symptoms of HF. IV Unable to carry on any physical activity without symptoms of HF, or symptoms of HF at rest. Etiology The most common cause of HF is left ventricular dysfunction due to hypertension in approximately 60 to 80 percent of the American population.1 However, impairments of the pericardium, myocardium, endocardium, heart valves or metabolic abnormalities are other underlying conditions that may lead to HF.1 The causes of HFrEF and HFpEF somewhat differ. Approximately two-thirds of HFrEF cases arise due to coronary artery disease (CAD), likely complicated by hypertension and/or diabetes.4 Underlying causes of HFrEF can be divided into ischemic and nonischemic cardiomyopathies. Myocardial infarction (MI) is a major cause of ischemic cardiomyopathy. An exhaustive list of syndromes that have the potential to develop into nonischemic cardiomyopathies include familial or idiopathic causes, obesity, diabetes, thyroid disease, acromegaly and growth hormone deficiency, excessive alcohol consumption, cocaine use, cancer therapy, myocardial toxins (e.g., ephedra, amphetamine, anabolic steroids), tachycardiainduced, myocarditis, HIV, Chagas disease, hypersensitivity myocarditis, rheumatological and connective tissue disorders, peripartum, iron overload, amyloidosis, cardiac sarcoidosis and Takotsubo syndrome.1 Conversely, older, obese women and individuals with atrial fibrillation and hypertension are more likely to develop HFpEF.5,6 Risk Factors Unfortunately, adults 40 years of age or older have a 20 percent lifetime risk of developing HF.7 More than five million people suffer from this disease presently.8 The prevalence of each type of HF is equally divided at 50 percent in the general population.9 Those having the highest risk of developing HF are individuals who are 65 years of age or older.10 In particular, African American males are at a higher risk of developing HF, compared to Caucasian women having the lowest risk.1114 Prevention is of utmost importance, as the mortality rate within five years of diagnosis is estimated to be 50 percent, as aforementioned.12,13 Hypertension, diabetes, metabolic syndrome and atherosclerosis are amongst the most important modifiable risk factors of HF. 16-20 Long-term treatment of these comorbid conditions can significantly reduce this risk up to 50 percent.21-24 Pathophysiology As earlier described, HF is best characterized as a progressive disease. Changes that occur to the myocardial anatomy, specifically the left ventricle, are a result of myocyte death and neurohormonal activation.25,26 Compensatory mechanisms following myocardium injury lead to reconstruction of the heart’s anatomy in a phenomenon known as remodeling, causing dilation of the ventricle, hypertrophy and reduced contractility, and decline in systolic function.25,26 The reninangiotensin-aldosterone system (RAAS) and sympathetic nervous system (SNS) are activated as the heart attempts to restore hemodynamic stability. In the RAAS, renin, located in the juxtaglomerular cells of the renal afferent arteriole, is cleaved by prorenin.27 Baroreceptors in the afferent arteriole walls are augmented by reduced perfusion pressure and systemic volume causing renin release. 28 Angiotensinogen forms into angiotensin I via cleavage of renin, subsequently leading to the activation of angiotensin II via ACE. Alternatively, angiotensin II can also be converted from angiotensin I by protease chymase in an alternative pathway.29 Angiotensin II responds to systemic volume depletion and lowered blood pressure while maintaining cardiovascular homeostasis. 30 Local production of angiotensin has been located in numerous tissues, including the heart.31,32 Of note, a negative feedback mechanism is present in response to increased angiotensin II levels. Angiotensin II activates several cardiovascular and renal mechanisms through angiotensin I receptors, including systemic arterial vasoconstriction, renal arteriolar vasoconstriction, stimulation of renal tubular reabsorption of sodium and water, vascular smooth muscle contraction, cellular proliferation, oxidative stress and aldosterone release from the adrenal glands.27 Stimulation of the angiotensin I receptor by angiotensin II produces cellular and fibroblast hypertrophy and collagen deposition leading to myocardial fibrosis in the myocardium.33,34 Hypertrophy and fibrosis promote ventricular remodeling, a hallmark sign of HFrEF. Aldosterone stimulates collagen synthesis and increases the level of ACE in the heart, further increasing angiotensin II production.35,36 The SNS is responsible for heart rate acceleration, cardiac contractility, reduced venous capacitance and peripheral vasoconstriction via cardiac sympathetic nerve fibers in the coronary arteries, mainly in the ventricles.37 Once the SNS is activated, norepinephrine and epinephrine is released. Norepinephrine increases contractile strength and blood pressure in the left ventricle, and increases heart rate and shortens atrioventricular conduction by traveling through the sinus and atrioventricular nodes. Locally released epinephrine and norepinephrine exert effects on the myocardium and peripheral vessels.37 In HFrEF, SNS is hyperactive as a result of cardiovascular reflex abnormalities.38,39 The inhibitory SNS arterial baroreceptor reflex is significantly diminished; however, the excitatory peripheral chemoreceptor reflex is amplified.40 The activation of the RAAS and SNS is initially beneficial, but prolonged activation leads to cardiac and peripheral hemodynamic instability. 41 This leads to harmful effects on, but not limited to, the heart, blood vessels, kidneys, muscles, bone marrow, lungs and liver. Further damage continues in a positive feedback fashion leading to symptomatology and deficiency within the framework of the myocardium, including electrical abnormalities. 25,26 As a result, a decreased quality of life, decrease in the functional capacity of the heart, decompensation, increase in hospitalizations and premature death from pump failure or ventricular arrhythmia occurs. 2 Pharmacological therapy, such as ACE inhibitors and BB aim to halt the cycle created by this detrimental environment by working on RAAS and SNS. ACE Inhibitors and Landmark HFrEF Trials As the name implies, ACE inhibitors block the transformation of angiotensin I into angiotensin II by decreasing the production of the ACE enzyme. These agents also increase nitric oxide stimulation, causing positive outcomes on endothelial function by halting the breakdown of bradykinin.42 Additionally, cardiac hemodynamics and exercise capacity are improved by decreasing systemic vascular resistance.43,44 However, it is important to keep in mind that not all patients will tolerate these agents. Common side effects include hypotension, dizziness and dry cough. Monitoring for severe adverse events, including angioedema and anaphylactic reactions, must also be taken into consideration.45 There are several landmark trials that have demonstrated the impact of ACE inhibitors in HF patients starting with CONSENSUS.46 This study looked at patients with New York Heart Association (NYHA) class IV symptoms with a heart size greater than 550 mL/m2 (women) and 600 mL/m2 (men) receiving enalapril compared to placebo for a mean of 6.2 months. Patients were randomized to enalapril 5 mg twice daily and, as tolerated, were then increased to 10 mg twice daily after one week up to a goal (target) dose of 20 mg twice daily. Patients had to be on a diuretic and digoxin for at least two weeks. Other medications such as spironolactone, BB and vasodilators were allowed to help control symptoms. Of note, only 3 percent of patients were on concomitant BB treatment, yet 52 percent were also taking spironolactone. The primary endpoint was six-month mortality. The secondary endpoint was 12-month and overall mortality. Originally planned to continue for several years, the Ethical Review Committee observed a consistent mortality difference in favor of enalapril after a year and a half, which ended the trial early. The mortality benefits observed were significantly different compared to placebo, showing a 40 percent relative risk reduction at six months (p = 0.002), 31 percent at one year (p = 0.001) and 27 percent reduction in total mortality over the entire study period (p = 0.003). Furthermore, cardiac death (including death from renal-artery thrombosis, endocarditis, pulmonary emboli after leg amputation, bronchitis and concomitant HF, occlusion of femoral arterial graft and HF in relation to melena) was significantly reduced compared to placebo (p = 0.001) as well as progression of congestive HF (p = 0.001). Additionally, heart size was significantly reduced in the enalapril group (p = 0.02). This investigation showed that enalapril in patients with severe HF can reduce mortality and improve symptoms. This was the first trial to demonstrate a mortality benefit of ACE inhibition in HF. CONSENSUS proved that ACE inhibitors were useful in severe HF patients; however, effectiveness in patients with NYHA class I, II and III symptoms was still unknown. Subsequently, the SOLVD trial aimed to answer this question. This trial was separated into the SOLVD treatment trial for patients with overt HF, and the SOLVD prevention trial for patients without HF symptoms.47,48 For both trials, patients diagnosed with congestive HF with an LVEF less than 35 percent were included. Patients received either enalapril 2.5 to 5 mg twice daily that was then titrated, if tolerated, to a target dose of 10 mg twice daily or placebo for a mean of 41 months. The primary endpoint was all-cause mortality. Secondary endpoints included hospitalization for congestive HF, incidence of MI, mortality due to specific causes, and a combined analysis of morbidity and mortality. In the SOLVD treatment trial, 57 percent of patients had class II symptoms, 30 percent had class III symptoms and 11 percent had class I symptoms. Patients were allowed to receive other HF agents, including digoxin, diuretics, vasodilators and calcium channel blockers. Only 8 percent of patients were also receiving a BB. No patients received spironolactone. A significant difference in all-cause mortality was found in favor of enalapril, observed to be most marked in the first 24 months with a risk reduction (RR) of 16 percent (RR 0.16; 95 percent CI, 0.05 to 0.26; p < 0.0036). A significant difference in favor of enalapril also appeared when comparing deaths or hospitalization for CHF (RR 0.26; 95 percent CI, 0.18 to 0.34; p < 0.0001), cardiovascular death (RR 0.18; 95 percent CI, 0.06 to 0.28; p < 0.002), cardiac events (RR 0.19; 95 percent CI, 0.07 to 0.29; p < 0.0015), and HF or arrhythmia with CHF (RR 0.22; 95 percent CI, 0.06 to 0.35; p < 0.0045). A nonsignificant positive trend for reduction in MI with enalapril use was also observed (RR 0.28; 95 percent CI, -0.08 to 0.52; p < 0.07). A significant reduction in mortality and hospitalizations for CHF in patients treated with an ACE inhibitor and conventional therapy was further established. This added to the results provided by CONSENSUS by demonstrating a 16 percent reduction in the risk of death with an ACE inhibitor in patients with an LVEF less than 35 percent with symptoms associated with any NYHA class. The SOVLD prevention trial looked at asymptomatic patients who were not receiving HF treatment. All-cause mortality was not significantly different between treatment and placebo groups (RR 0.08; 95 percent CI, -0.08 to 0.21; p = 0.30). There was a larger but nonsignificant reduction in mortality from cardiovascular causes in those receiving enalapril (RR 0.12; 95 percent CI, -0.03 to 0.26; p = 0.12). When patients in whom HF developed and those who died were combined, the total number of deaths and cases of HF was significantly less in the enalapril group (RR 0.29; 95 percent CI 0.21 to 0.36; p < 0.001). In addition, fewer patients given enalapril died or were hospitalized for HF (RR 0.20; 95 percent CI, 0.09 to 0.30; p < 0.001). This trial showed that ACE inhibitors can significantly reduce the incidence of HF and rate of related hospitalizations in asymptomatic individuals with left ventricular dysfunction. There was also a trend toward fewer cardiovascular deaths in patients who received enalapril. Before ACE inhibitors and BB, vasodilators were the mainstay of HF treatment. The VHeFT II trial compared the then standard therapy to the emerging ACE inhibitor therapy. 49 Men with a LVEF of less than 45 percent and reduced exercise tolerance were followed for a mean of 2.3 years. Patients received enalapril 20 mg daily or hydralazine 300 mg plus isosorbide dinitrate 160 mg daily. Mortality by any cause was the primary endpoint. Secondary endpoints were not specified, but factors such as mortality for each year studied, change in LVEF and exercise capacity by looking at oxygen consumption were reviewed. No patients were on concomitant BB or spironolactone therapy. Results showed a nonsignificant favorable trend in patients receiving enalapril regarding allcause mortality over the entire study course (p = 0.08). However, mortality after two years was significantly less in the enalapril arm (p = 0.016). After the first year of treatment, enalapril reduced mortality by 13 percent and hydralazine/isosorbide dinitrate by 9 percent. This increased over time, as enalapril reduced mortality by 54 percent and hydralazine/isosorbide dinitrate by 48 percent over five years. Sudden cardiac death occurred significantly less in the enalapril group whether with (p = 0.032) or without warning (p = 0.015) of an event. Left ventricular ejection fraction after 13 weeks and annually after randomization were significantly increased in both treatment groups for three years (p = 0.0001) with the increased LVEF after 13 weeks being significantly greater in the hydralazine/isosorbide dinitrate group (p = 0.026). Oxygen consumption was shown to be increased significantly by hydralazine/isosorbide dinitrate after 13 weeks (p < 0.0001) and after six months (p < 0.0001), but not enalapril. The transverse diameter of the heart was also found to be significantly reduced in both treatment arms after 13 weeks and one year (p < 0.0001). There was no significant difference amongst patients in either group. Based on these results, the investigators suggested using the hydralazine/isosorbide dinitrate combination agent with ACE inhibitor therapy for further mortality benefit and symptom relief in HF. Overall, this trial revealed ACE inhibitor superiority over hydralazine plus nitrate combination therapy in HF patients. STOP AND REFLECT A 60-year-old Caucasian male presents to your clinic with a new HF diagnosis. His LVEF is 20 percent and he is exhibiting dyspnea on exertion and +1 pitting edema. His current HF failure regimen includes furosemide 20 mg daily and hydralazine 300 mg/isosorbide dinitrate 160 mg, one tablet daily. What medication changes should you consider in this patient? Beta-blockers and Landmark HFrEF Trials BB inhibit the binding of norepinephrine and epinephrine to beta receptors located in the heart, bronchial and vascular musculature. Properties of BB that are beneficial in HF include decreased sympathetic outflow from the vasomotor centers of the brain, and the inhibition of renin release by the kidneys.50 In total, three BB have proven to be effective in mortality reduction among patients with HFrEF – bisoprolol, carvedilol and metoprolol succinate. Both bisoprolol and metoprolol succinate are selective for beta-1 receptors, although this selectivity may be lost at higher doses. Metoprolol succinate maintains its beta-1 selectivity until a maintenance dose of 200 mg is reached, and bisoprolol maintains its beta-1 selectivity until a maintenance dose of 20 mg daily is reached, which exceeds the target dose in HFrEF.50,51 Carvedilol blocks alpha-1, beta-1 and beta-2 receptors.52 An important concept to remember when initiating BB in HF is the potential for fluid retention and worsening symptoms of HF. For this reason, BB should be initiated at low doses, and increased gradually to their target doses. If fluid retention does occur, a patient’s diuretic dose should be increased, and up-titration of the BB dose should be postponed until the patient is clinically stable.52 In some cases such as when patients are unresponsive to diuretic therapy or patients require the use of inotropes, it may be useful to temporarily lower the dose or discontinue the BB.53 Of note, worsening HF symptoms with a BB should not be a reason to avoid BB permanently. Additional adverse effects with BB include fatigue, bradycardia, heart block and hypotension. Multiple trials have demonstrated the utility of BB in HFrEF. The U.S. Carvedilol Heart Failure Study Group trial was the first trial to demonstrate a mortality benefit with BB in the treatment of HF.54 This trial included patients with symptoms of HF for at least three months, a LVEF ≤ 35 percent, and two or more months of treatment with diuretics and an ACE inhibitor. Patients were randomized to carvedilol 6.25 mg twice daily or placebo, with a gradual dose increase to 25 to 50 mg twice daily over two to 10 weeks, as tolerated. The mean total daily dose of carvedilol was 45 mg. The primary outcome was all-cause mortality. Treatment with carvedilol led to a 65 percent relative risk reduction for death as compared to placebo (p < 0.001). In addition, carvedilol led to fewer hospitalizations due to cardiovascular causes (RR 0.73; 95 percent CI, 0.55-0.97; p = 0.036) and a decrease in the combined risk of death or hospitalization due to cardiovascular reasons (HR 0.62; 95 percent CI, 0.47-0.82; p < 0.001). In this trial, 95 percent of patients also received an ACE inhibitor at baseline. This was the first trial to demonstrate the utility and benefit of BB therapy in HF patients. Next, the COPERNICUS trial enrolled patients with severe symptomatic chronic HF, defined as dyspnea or fatigue at rest or on minimal exertion for two months, a LVEF < 25 percent despite optimal medical therapy and clinical euvolemia.55 Patients were randomized to carvedilol 3.125 mg twice daily or placebo. Doses were doubled every two weeks to achieve a target dose of 25 mg twice daily, as tolerated. The mean daily dose of carvedilol achieved in COPERNICUS was 37 mg. Patients were evaluated for a primary outcome of all-cause mortality, in which carvedilol demonstrated a 35 percent relative risk reduction compared to placebo (p = 0.00013). A secondary outcome in COPERNICUS was the combined risk of death or all-cause hospitalization. For this outcome, carvedilol demonstrated a 35 percent relative risk reduction compared to placebo (p < 0.001). In addition to carvedilol or placebo, 97 percent of patients received an ACE inhibitor or angiotensin II receptor blocker (ARB), and 20 percent of patients received spironolactone at baseline. COPERNICUS further highlighted the mortality benefits as well as decrease in hospitalization that BB therapy offered to the HF population. CIBIS-II included patients with NYHA class III-IV symptoms of HF, and a LVEF ≤ 35 percent.56 Heart failure was diagnosed at least three months prior to enrollment. Additionally, patients needed to be clinically stable for at least six weeks for HF symptoms, and three months for unstable angina or MI. Study participants were randomized to bisoprolol 1.25 mg daily or placebo, with dose titrations to a goal dose of 10 mg daily. This dose was achieved in 43 percent of patients during the study. Ninety six percent of patients were also taking an ACE inhibitor during this trial. The primary outcome was all-cause mortality. Secondary outcomes included all-cause hospital admissions, cardiovascular mortality, the composite of cardiovascular mortality and cardiovascular hospital admissions, and permanent premature treatment withdrawal. Patients were followed for a mean of 1.3 years, and the trial was stopped early due to a 32 percent risk reduction in all-cause mortality with bisoprolol as compared to placebo (p < 0.0001). In addition, bisoprolol decreased allcause hospitalizations (HR 0.80; p = 0.0006), cardiovascular mortality (HR 0.71; p = 0.0049), and cardiovascular mortality and cardiovascular hospital admissions (HR 0.79; p = 0.0004). CIBIS II revealed that bisoprolol was another option for BB therapy in HF patients, which also reduced morbidity and mortality as well as hospitalizations. The MERIT-HF trial compared metoprolol succinate 12.5 mg or 25 mg daily to placebo in the reduction of all-cause mortality among HF patients for a mean follow-up of one year.57 The metoprolol dose was doubled every two weeks to a target of 200 mg daily, as tolerated. Inclusion criteria for this trial was patients with NYHA class II-IV HF for at least three months with an LVEF ≤ 40 percent within three months. Patients needed to be on optimum therapy with an ACE inhibitor and diuretic. This trial demonstrated a 34 percent relative risk reduction in all-cause mortality with metoprolol succinate (p = 0.00009), and led to its FDA approval in HF management. This study also demonstrated a significant reduction in all-cause mortality plus all-cause hospitalization (RR 0.19; 95 percent CI, 0.10-0.27; p < 0.001), all-cause mortality or HF hospitalization (RR 0.31; 95 percent CI 0.20-0.40; p < 0.001), death or heart transplant (RR 0.32; 95 percent CI, 0.16-0.45; p < 0.001), cardiac death or nonfatal MI (RR 0.39; 95 percent CI, 0.25-0.51; p < 0.001), and all-cause mortality or all-cause hospitalization or ED visit for HF (RR 0.32; 95 percent CI, 0.21-0.41; p < 0.001) with metoprolol as compared to placebo. At baseline, 89 percent of patients enrolled in the trial received an ACE inhibitor, and 7 percent received an ARB. With the positive results of MERIT-HF, metoprolol succinate joined carvedilol and bisoprolol in effective BB agents to prevent morbidity and mortality in HF. Table 2. Landmark Trials of ACE inhibitors and Beta-blockers Trial Agents Primary Results Outcomes CONSENSUS 1. Enalapril 5-20 mg Six-month Six-month mortality: 26 percent vs. twice daily mortality 44 percent (RR 0.60; p = 0.002) (as tolerated) SOLVD-T SOLVD-P V-HeFT CIBIS II 2. Placebo 1. Enalapril 2.5-10 mg twice daily (as tolerated) 2. Placebo 1. Enalapril 20 mg daily 2. Hydralazine 300 mg/Isosorbide dinitrate 160mg daily 1. Bisoprolol 1.2510mg daily (as tolerated) 2. Placebo COPERNICUS 1. Carvedilol 3.125-25 mg twice daily (as tolerated) MERIT-HF All-cause mortality Two-year mortality and overall mortality 35 percent vs. 40 percent (RR 0.84; 95 percent CI 0.74-0.95; p = 0.0036) 14.8 percent vs. 15.8 percent (RR 0.08; 95 percent CI, -0.08 to 0.21; p = 0.30) Two-year mortality: 18 percent vs. 25 percent (p=0.016) Overall mortality: 132 events vs. 153 events (p = 0.08) All-cause mortality All-cause mortality: 11.8 percent vs. 17.3 percent p<0.0001) All-cause mortality All-cause mortality: 11.2 percent vs. 16.8 percent (p = 0.00013) 2. Placebo 1. Metoprolol succinate All-cause 12.5-200 mg daily mortality and all-cause 2. Placebo mortality plus all-cause hospitalization All-cause mortality: 7.2 percent vs. 11 percent (p = 0.00009) All-cause mortality plus all-cause hospitalization: 32 percent vs. 38 percent (p < 0.001) Table 3. Pharmacology and Target Doses of ACE Inhibitors Drug Half-life Onset of Dose Range and (Hours) Action Frequency (Hours) Captopril 2 0.25-0.5 6.25-150 mg thrice daily Enalapril 11 1-2 2.5-10 mg twice daily Lisinopril 12 1-2 5-20 mg daily Ramipril 16 1-2 2.5-10 mg daily or in divided doses Quinapril 25 1-2 5-40 mg daily or in divided doses Fosinapril 12 1-2 5-20 mg daily Table 4. Pharmacology and Target Doses of Beta-blockers Drug Half-life Onset of (Hours) Action (Hours) Bisoprolol 9-12 1-2 Carvedilol 7-10 1-2 Metoprolol Succinate 3-4 1-2 12.5-200 mg daily Target Dose for Survival Benefit 50 mg thrice daily 10 mg twice daily 10-20 mg daily 5 mg daily 20 mg twice daily 20 mg daily Dose Range and Frequency 1.25-10 mg daily 3.125-50 mg twice daily Target Dose for Survival Benefit 10 mg daily 25 mg twice daily (< 85 kg) 50 mg twice daily (> 85 kg) 200 mg daily STOP AND REFLECT Three months later, the same 60-year-old Caucasian male returns to your clinic for optimization of HF management. His current HF regimen includes furosemide 20 mg daily, lisinopril 20 mg daily and hydralazine 300 mg/isosorbide dinitrate 160 mg, one tablet daily. He is euvolemic on examination. What medication changes should you consider in this patient? Guideline Recommendations As a result of the described landmark trials, the 2013 ACCF/AHA guidelines recommend the use of ACE inhibitors (Level of Evidence A) and BB (Level of Evidence B) in all individuals with a history of MI or acute coronary syndrome (ACS) and reduced LVEF with evidence of structural heart abnormalities.1 In patients without a history of MI, ACE inhibitors (Level of Evidence A) and BB (Level of Evidence C) are still recommended to prevent symptomatic HF and reduce mortality.1 The ESC 2012 guidelines also recommend the use of ACE inhibitors and BB and suggest that both be started as soon as possible after diagnosis due to their mortality benefits as well as ACE inhibitors effects on remodeling and improvement in LVEF by BB.2 Of note, in the suggested treatment algorithm of the ESC guideline, ACE inhibitors are initiated with diuretics first, then BB are subsequently added to therapy. Special Considerations Both ACE inhibitors and BB have demonstrated mortality benefit in HFrEF. It is recommended that both classes of medications be initiated in HFrEF. When determining which agent to initiate first, it is important to consider patient-specific factors and comorbidities. Pregnancy/Lactation/Women One factor to consider when choosing a HF strategy is pregnancy. A boxed warning exists for ACE inhibitors, stating that they should be discontinued as soon as possible when pregnancy is detected.45 This is because drugs acting on the RAAS can cause injury or death to a developing fetus. As a result, women, especially those of child-bearing age, are less inclined to receive ACE inhibitor therapy. In cases of HF and pregnancy, a BB would be the safest option for management. ACE inhibitors are also excreted in breast milk; breast-feeding while on ACE inhibitor therapy is not recommended by the manufacturer.45 Caution in breast-feeding is also recommended with BB use.5052 Symptomatic Heart Failure As previously mentioned, BB have the potential to increase fluid retention and worsen the symptoms of HF.50,52 While this effect does not exclude a patient from the initiation of a BB, it may temporarily limit the ability to titrate a BB to a target dose. This effect is not seen with ACE inhibitors, so an ACE inhibitor may be a preferable initial agent for a patient hospitalized with symptoms of HF. Myocardial Infarction/Angina Both ACE inhibitors and BB are recommended to be initiated post MI for secondary prevention.58 Additionally, BB have utility for the management of angina. In a patient with HF and complaints of chest pain, increasing the BB dose may be the best first option. Atrial Fibrillation Atrial fibrillation (AF) is an irregular heart beat characterized by uncoordinated atrial activation and ineffective atrial contraction.59 Patients may experience symptoms, including fatigue, palpitations, dyspnea, syncope, and development or worsening of HF. A BB can be utilized for rate control and may alleviate such symptoms. Some data suggests that bucindolol may be beneficial in patients with AF and HF; additional research is warranted.60 Currently, carvedilol, metoprolol succinate and bisoprolol remain the only BBs indicated for HFrEF. Ventricular Arrhythmias Ventricular arrhythmias range in severity from being asymptomatic, to causing sudden death, and include conditions such as premature ventricular complexes, ventricular tachycardia, and ventricular flutter and fibrillation.61 BB are recommended for the suppression of ventricular ectopic beats and arrhythmias and have shown efficacy in the reduction of sudden cardiac death among patients with and without HF. Additionally, carvedilol may be superior to metoprolol in the prevention of ventricular arrhythmias.62 Among patients prescribed BB therapy in the Multicenter Automatic Defibrillator Implantation Trial with Cardiac Resynchronization Therapy (MADITCRT), ventricular arrhythmias occurred in 26 percent of patients prescribed metoprolol, and 22 percent of patients prescribed carvedilol (HR 0.80; 95 percent CI: 0.63 to 1; p = 0.05).62 Diabetes/Chronic Kidney Disease ACE inhibitors demonstrate protective effects on the kidneys and may be especially helpful in patients with diabetes or chronic kidney disease. These protective effects partially are a result of antihypertensive mechanisms. Additionally, intrarenal efferent vasodilation leads to a fall in filtration pressure contributing to beneficial effects.63 Of note, use of an ACE inhibitor can cause acute kidney injury, and should be used with caution in patients with a history of fluctuating renal function. In terms of BB therapy, carvedilol may be a better option for HF patients with concomitant diabetes. Compared to metoprolol, carvedilol does not blunt insulin sensitivity and has not been shown to worsen diabetes control as measured by HbA1C.64,65 Restrictive Lung Disease Beta-receptors are located throughout the body, including beta-2 receptors found in the lungs. In patients with restrictive lung disease, utilization of a BB may exacerbate breathing problems. Although metoprolol and bisoprolol are selective to beta-1 receptors in the heart, keep in mind they may lose their selectivity at higher doses as mentioned previously.50,51 On the other hand, bisoprolol is the most beta-1 selective agent, and retains beta-1 selectivity at its target dose for HF, 10 mg daily.1,51 ACE inhibitors have no effect on the beta receptors, and may be a better option in such patients. However, keep in mind the potential of ACE-induced coughing.45 Asian/African Americans and ACE-induced Cough A common side effect of ACE inhibitors is a dry cough, which may lead to a discontinuation of the medication. Studies have shown that an ACE inhibitor-induced cough is more common among black and Asian patients.66,67 Thus, ethnicity is an important factor to consider when initiating medications for the management of HF. In black and Asian patients, it may be reasonable to initiate an ARB as an alternative to an ACE inhibitor if a history of dry cough exists. Conclusion Heart failure is a progressive disease marked by any structural or functional impairment of ventricular filing or ejection of blood. First-line agents for this condition include ACE inhibitors and BB, as several landmark trials have demonstrated a benefit in morbidity and mortality as well as a reduction in hospitalizations. To our knowledge, only one large, randomized, controlled trial aimed to find whether ACE inhibitors or BB should be initiated first in HFrEF by comparing bisoprololfirst to enalapril-first treatment. The primary endpoint, all-cause mortality or hospitalization, was found to be similar between both drug classes (absolute difference -1.6 percent; 95 percent CI, 0.076 to 0.044; HR 0.94; 95 percent CI, 0.77-1.16), indicating that ACE inhibitors and BB are equally safe and efficacious to initiate first.68 European guidelines favor the use of ACE inhibitors first when utilizing a step-wise approach for initiating first-line therapy. In comparison, the United States guidelines do not specify the use of one agent over another. With this said, clinicians should use their clinical judgment and take into account patient-specific characteristics when deciding whether to utilize an ACE inhibitor or BB first when starting HF therapy. Beta-blockers may be preferable in HF patients who are pregnant, or have a history of angina or arrhythmias. ACE inhibitor therapy may be preferable in symptomatic HF patients, patients with diabetes or chronic kidney disease, or in lung disease. Despite which agent is utilized first, the goal is to include both agents in the medication regimen in order to maximize morbidity and mortality benefits among HF patients. Continuing Education Self-assessment Questions 1. Which of the following distinguishes HFrEF from HFpEF? a. Evidence of left ventricular diastolic dysfunction b. A left ventricular ejection fraction of less than 40 percent c. A left ventricular ejection fraction of greater than 50 percent d. Evidence of a left ventricular thrombus 2. What is the most common cause/risk factor in developing heart failure? a. Hypertension b. Arrhythmia c. Sleep apnea d. Myocardial infarction 3. Which compensatory mechanisms are harmful to the myocardium resulting in HF if stimulated over a prolonged period of time? a. Sympathetic nervous system (SNS) b. Parasympathetic nervous system (PNS) c. Renin-angiotensin-aldosterone system (RAAS) d. Both A and C 4. Which landmark trial demonstrated a 31 percent risk reduction in mortality over one year when using ACE inhibitor treatment in patients with NYHA class IV symptoms? a. SOLVD treatment b. SOLVD prevention c. CONSENSUS d. V-HeFT II 5. Which landmark trial demonstrated a 16 percent risk reduction in mortality when using ACE inhibitor treatment in patients with any type of NYHA symptoms? a. SOLVD treatment b. SOLVD prevention c. CONSENSUS d. V-HeFT II 6. Which landmark trial demonstrated a 35 percent risk reduction in mortality when using BB treatment in patients with a LVEF less than 25 percent? a. MERIT HF b. COPERNICUS c. CIBIS-II d. US Carvedilol Trial 7. Which trial demonstrated mortality benefit with patients randomized to bisoprolol? a. MERIT HF b. COPERNICUS c. CIBIS-II d. US Carvedilol Trial 8. A 48-year-old female with HF and a LVEF of 25 percent presents to clinic two weeks after the initiation of metoprolol succinate 25 mg daily. Today, she complains of dyspnea on exertion, and a 7-pound weight gain within the past five days. Additional medications include lisinopril 10 mg daily and furosemide 20 mg daily. What medication change would you recommend at this time? a. Discontinue lisinopril b. Switch patient to metoprolol tartrate 25 mg twice daily c. Increase furosemide to 40 mg daily d. Increase metoprolol succinate to 50 mg daily 9. In which patient would you consider initial HF management with an ACE inhibitor? a. 25-year-old pregnant female with LVEF 35 percent b. 56-year-old male with a history of diabetes and gout c. 62-year-old female with complaints of chest pain on exertion d. 70-year-old male with ICD placement for supraventricular tachycardia 10. In which patient would you consider initial HF management with a BB? a. 48-year-old male with diabetes and proteinuria b. 36-year-old female presenting with pitting edema and shortness of breath c. 58-year-old male with atrial fibrillation with rapid ventricular rate d. 64-year-old male with severe COPD References: 1. Yancy, C.W., Jessup, M., Bozhurt, B., et al., 2013 ACCF/AHA Guidelines for the management of heart failure: A report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines, Circulation, 2013, 128, pp. e240-e327. 2. McMurray, J.J.V., Adamopoulous, S., Anker, S.D., Auricchio, A., et al., ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure 2012: The Task Force for the Diagnosis and Treatment of Acute and Chronic Heart Failure 2012 of the European Society of Cardiology, Developed in collaboration with the Heart Failure Association (HFA) of the ESC, European Heart Journal, 2012, 33, pp. 1787-1847. 3. Bhuiyan, T., Maurer, M.S., Heart failure with preserved ejection fraction: Persistent diagnosis, therapeutic enigma, Current Cardiovascular Risk Reports, 2011, 5, pp. 440-449. 4. Ackerman, M.J., Priori, S.G., Willems, S., Berul, C., Brugada, R., Calkins, H., Camm, A.J., Ellinor, P.T., Gollob, M., Hamilton, R., Hershberger, R.E., Judge, D.P., Le Marec, H., McKenna, W.J., Schulze-Bahr, E., Semsarian, C., Towbin, J.A., Watkins, H., Wilde, A., Wolpert, C., Zipes, D.P., HRS/EHRA Expert Consensus Statement on the State of Genetic Testing for the Channelopathies and Cardiomyopathies: This Document was Developed as a Partnership between the Heart Rhythm Society (HRS) and the European Heart Rhythm Association (EHRA), Heart Rhythm, 2011, 8, pp. 1308-1339. 5. Hogg, K., Swedberg, K., McMurray, J., Heart Failure with Preserved Left Ventricular Systolic Function; Epidemiology, Clinical Characteristics, and Prognosis, Journal of the American College of Cardiology, 2004, 43, pp. 317-327. 6. Lam, C.S., Donal, E., Kraigher-Krainer, E., Vasan, R.S., Epidemiology and Clinical Course of Heart Failure with Preserved Ejection Fraction, European Journal of Heart Failure, 2011, 13, pp. 18-28. 7. Djousse, L., Driver, J.A., Gaziano, J.M., Relation Between Modifiable Lifestyle Factors and Lifetime Risk of Heart Failure, The Journal of the American Medical Association, 2009, 302, pp. 394-400. 8. Go, A.S., Mozaffarian, D., Roger, V.L., et al., Heart Disease and Stroke Statistics–2013 Update: A report from the American Heart Association, Circulation, 2013, 127, pp. e6-245. 9. Owan, T.E., Hodge, D.O., Herges, R.M., et al., Trends in Prevalence and Outcome of Heart Failure with Preserved Ejection Fraction, New England Journal of Medicine, 2006, 355, pp. 251259. 10. The Booming Dynamics of Aging: From Awareness to Action, The White House Conference on Aging, Washington, D.C.: U.S. Department of Health and Human Services, 2011. 11. Curtis, L.H., Whellan, D.J., Hammill, B.G., et al., Incidence and Prevalence of Heart Failure in Elderly Persons, 1994-2003, Archives of Internal Medicine, 2008, 168, pp. 418-424. 12. Roger, V.L., Weston, S.A., Redfield, M.M., et al., Trends in Heart Failure Incidence and Survival in a Community-based Population, The Journal of the American Medical Association, 2004, 292, pp. 344-350. 13. Bahrami, H., Kronmal, R., Bluemke, D.A., et al., Differences in the Incidence of Congestive Heart Failure by Ethnicity: The Multi-ethnic Study of Atherosclerosis, Archives of Internal Medicine, 2008, 168, pp. 2138-2145. 14. Lloyd-Jones, D.M., Larson, M.G., Leip, E.P., et al., Lifetime Risk for Developing Congestive Heart Failure: The Framingham Heart Study, Circulation, 2002, 106, pp. 3068-3072. 15. Levy, D., Kenchaiah, S., Larson, M.G., et al., Long-term Trends in the Incidence of and Survival with Heart Failure, New England Journal of Medicine, 2002, 347, pp. 1397-1402. 16. Levy, D., Larson, M.G., Vasan, R.S., et al., The Progression from Hypertension to Congestive Heart Failure, The Journal of the American Medical Association, 1996, 275, pp. 15571562. 17. Wilhelmsen, L., Rosengren, A., Eriksson, H., et al., Heart Failure in the General Population of Men: Morbidity, Risk Factors and Prognosis, Journal of Internal Medicine, 2001, 249, pp. 253-261. 18. He, J., Ogden, L.G., Bazzano, L.A., et al., Risk Factors for Congestive Heart Failure in U.S. Men and Women: NHANES I Epidemiologic Follow-up Study, Archives of Internal Medicine, 2001, 161, pp. 996-1002. 19. Grundy, S.M., Cleeman, J.I., Merz, C.N., et al., Implications of Recent Clinical Trials for the National Cholesterol Education Program Adult Treatment Panel III Guidelines, Journal of the American College of Cardiology, 2004, 44, pp. 720-732. 20. Smith, S.C., Jr., Benjamin, E.J., Bonow, R.O., et al., AHA/ACCF Secondary Prevention and Risk Reduction Therapy for Patients with Coronary and Other Atherosclerotic Vascular Disease: 2011 Update: A Guideline from the American Heart Association and American College of Cardiology Foundation, Circulation, 2011, 124, pp. 2458-2473. 21. Effects of Treatment on Morbidity in Hypertension, II: Results in Patients with Diastolic Blood Pressure Averaging 90 through 114 mmHg, The Journal of the American Medical Association, 1970, 213, pp. 1143-1152. 22. Kostis, J.B., Davis, B.R., Cutler, J., et al., Prevention of Heart Failure by Antihypertensive Drug Treatment in Older Persons with Isolated Systolic Hypertension: SHEP Cooperative Research Group, The Journal of the American Medical Association, 1997, 278, pp. 212-216. 23. Izzo, J.L., Jr., Gradman, A.H., Mechanisms and Management of Hypertensive Heart Disease: From Left Ventricular Hypertrophy to Heart Failure, Medical Clinics of North America, 2004, 88, pp. 1257-1271. 24. Baker, D.W., Prevention of Heart Failure, Journal of Cardiac Failure, 2002, 8, pp. 333-346. 25. McMurray, J.J., Clinical Practice, Systolic Heart Failure, New England Journal of Medicine, 2010, 362, pp. 228-238. 26. Shah, A.M., Mann, D.L., In Search of New Therapeutic Targets and Strategies for Heart Failure: Recent Advances in Basic Science, Lancet, 2011, 378, pp. 704-712. 27. Sayer, G., Bhat, G., The Renin-angiotensin-aldosterone System and Heart Failure, Cardiology Clinics, 2014, 32, pp. 21-32. 28. Bock, H.A., Hermle, M., Brunner, F.P., et al., Pressure Dependent Modulation of Renin Release in Isolated Perfused Glomeruli, Kidney International, 1992, 41, pp. 275-280. 29. Urata, H., Healy, B., Steward, R.W., et al., Angiotensin II-forming Pathways in Normal and Failing Human Hearts, Circulation Research, 1990, 66, pp. 883-890. 30. Dzau, V.J., Tissue Renin-angiotensin System in Myocardial Hypertrophy and Failure, Archives of Internal Medicine, 1993, 153, pp. 937-942. 31. Seikaly, M.G., Arant, B.S., Seney, F.D., Endogenous Angiotensin Concentrations in Specific Intrarenal Fluid Compartments of the Rat, Journal of Clinical Investigation, 1990, 86, pp. 13521357. 32. van Kats, J.P., Danser, A.H., van Meegen, J.R., et al., Angiotensin Production by the Heart: A Quantitative Study in Pigs with the Use of Radiolabeled Angiotensin Infusions, Circulation, 1998, 98, pp. 73-81. 33. Peng, J., Gurantz, D., Tran, V., et al., Tumor Necrosis Factor-alpha-induced AT1 Receptor Upregulation Enhances Angiotensin II-mediated Cardiac Fibroblast Responses that Favor Fibrosis, Circulation Research, 2002, 91, pp. 1119-1126. 34. Weber, K.T., Extracellular Matrix Remodeling in Heart Failure: A Role for De Novo Angiotensin II Generation, Circulation, 1997, 96, pp. 4065-4082. 35. Brilla, C.G., Zhou, G., Matsubara, L., et al., Collagen Metabolism in Cultured Adult Rat Cardiac Fibroblasts: Response to Angiotensin II and Aldosterone, Journal of Molecular and Cellular Cardiology, 1994, 26, pp. 809-820. 36. Harada, E., Yoshimura, M., Yasue, H., et al., Aldosterone Induces Angiotensin-convertingenzyme Gene Expression in Cultured Neonatal Rat Cardiocytes, Circulation,2001, 104, pp. 137-139. 37. Zhang, D.Y., Anderson, A.S., The Sympathetic Nervous System and Heart Failure, Cardiology Clinics, 2014, 32, pp. 33-45. 38. Triposkiadis, F., Karayannis, G., Giamouzis, G., et al., The Sympathetic Nervous System in Heart Failure: Physiology, Pathophysiology and Clinical Implications, Journal of the American College of Cardiology, 2009, 54, pp. 1747-1762. 39. Floras, J.S., Sympathetic Nervous System Activation in Human Heart Failure: Clinical Implications of an Updated Model, Journal of the American College of Cardiology, 2009, 54, pp. 375-385. 40. Watson, A.M., Hood, S.G., May, C.N., Mechanisms of Sympathetic Activation in Heart Failure, Clinical and Experimental Pharmacology and Physiology, 2006, 33, pp. 1269-1274. 41. Kishi, T., Heart Failure as an Autonomic Nervous System Dysfunction, Journal of Cardiology, 2012, 59, pp. 117-122. 42. Hornig, B., Kohler, C., Drexler, H., Role of Bradykinin in Mediating Vascular Effects of Angiotensin-converting Enzyme Inhibitors in Humans, Circulation, 1997, 95, pp. 1115-1118. 43. Levine, T.B., Olivari, M.T., Garberg, V., et al., Hemodynamic and Clinical Response to Enalapril, a Long-acting Converting-enzyme Inhibitor, in Patients with Congestive Heart Failure, Circulation, 1984, 69, pp. 548-553. 44. Uretsky, B.F., Shaver, J.A., Liang, C.S., et al., Modulation of Hemodynamic Effects with a Converting Enzyme Inhibitor: Acute Hemodynamic Dose-Response Relationship of New Angiotensin Converting Enzyme Inhibitor, Lisinopril, with Observations on Long-term Clinical, Functional and Biochemical Responses, American Heart Journal, 1988, 116, pp. 480488. 45. Vasotec® [package insert], Bridgewater, NJ, Valeant Pharmaceuticals, Inc., 2011. 46. CONSENSUS Trial Study Group, Effects of Enalapril on Mortality in Severe Congestive Heart Failure: Results of the Cooperative North Scandinavian Enalapril Survival Study (CONSENSUS), New England Journal of Medicine, 1987, 316, pp. 1429-1435. 47. The SOLVD Investigators, Effect of Enalapril on Survival in Patients with Reduced Left Ventricular Ejection Fractions and Congestive Heart Failure, New England Journal of Medicine, 1991, 325, pp. 293-302. 48. The SOLVD Investigators, Effect of Enalapril on Mortality and the Development of Heart Failure in Asymptomatic Patients with Reduced Left Ventricular Ejection Fractions, New England Journal of Medicine, 1992, 327, pp. 685-691. 49. Cohn, J.N., Johnson, G., Ziesche, S., et al., A Comparison of Enalapril with HydralazineIsosorbide Dinitrate in the Treatment of Chronic Congestive Heart Failure, New England Journal of Medicine, 1991, 325, pp. 303-310. 50. Toprol XL® [package insert], Wilmington, DE. AstraZeneca, LP;2014. 51. Zebeta® [package insert], Pomona, NY, Duramed Pharmaceuticals, Inc., 2010. 52. Coreg® [package insert], Research Triangle Park, NC, Glaxo Smith Kline, LLC, 2013. 53. Alharethi, R., Hershberger, R.E., Beta-blocker Use in Decompensated Heart Failure, Current Heart Failure Reports, 2006, 3, pp. 75-80. 54. Packer, M., Bristow, M.R., Cohn, J.N., et al., The Effect of Carvedilol on Morbidity and Mortality in Patients with Chronic Heart Failure, U.S. Carvedilol Heart Failure Study Group, New England Journal of Medicine, 1996, 334, pp. 1349-1355. 55. Packer, M., Coats, A.J., Fowler, M.B., et al., Effect of Carvedilol on Survival in Severe Chronic Heart Failure, New England Journal of Medicine, 2001, 344, pp. 1651-1658. 56. The CIBIS-II Investigators, The Cardiac Insufficiency Bisoprolol Study II (CIBIS-II): A Randomized Trial, Lancet, 1999, 353, pp. 9-13. 57. The MERIT-HF Investigators, Effect of Metoprolol CR/XL in Chronic Heart Failure: Metoprolol CR/XL Randomized Intervention Trial in Congestive Heart Failure (MERITHF), Lancet, 1999, 353, pp. 2001-2007. 58. Smith, S.C., Benjamin, E.J., Bonow, R.O., et al., AHA/ACCF Secondary Prevention and Risk Reduction Therapy for Patients with Coronary and Other Atherosclerotic Vascular Disease: 2011 Update, Circulation, 2011, 124, pp. 2458-2473. 59. January, C.T., Wann, L.S., Alpert, J.S., et al., 2014 AHA/ACC/HRS Guideline for the Management of Patients with Atrial Fibrillation, Circulation, 2014, 130, pp. e199-e267. 60. Kao, D.P., Davis, G., Aleong, R., et al., Effect of Bucindolol on Heart Failure Outcomes and Heart Rate Response in Patients with Reduced Ejection Fraction and Atrial Fibrillation, European Journal of Heart Failure, 2013, 15, pp. 324-333. 61. Zipes, D.P., Camm, A.J., Borggrefe, M., et al., ACC/AHA/ESC 2006 Guidelines for Management of Patients with Ventricular Arrhythmias and the Prevention of Sudden Cardiac Death, Circulation, 2006, 114, pp. e385-e484. 62. Ruwald, M.H., Ruwald, A.C.H., Jons, C., et al., Effect of Metoprolol Versus Carvedilol on Outcomes in MADIT-CRT (Multicenter Automatic Defibrillator Implantation Trial With Cardiac Resynchronization Therapy), Journal of the American College of Cardiology, 2013, 61, pp. 1518-1526. 63. Navis, G., Faber, H.J., de Zeeuw, D., de Jong, P.E., ACE Inhibitors and the Kidney. A Riskbenefit Assessment, Drug Safety, 1996, 15, pp. 200-211. 64. Bakris, G.L., Fonseca, V., Katholi, R.E., et al., Metabolic Effects of Carvedilol vs Metoprolol in Patients With Type 2 Diabetes Mellitus and Hypertension: A Randomized Controlled Trial, The Journal of the American Medical Association, 2004, 292, pp. 2227-2236. 65. Kveiborg, B., Hermann, T.S., Major-Pederson, A., et al., Metoprolol Compared to Carvedilol Deteriorates Insulin-stimulated Endothelial Function in Patients with Type 2 Diabetes – A Randomized Study, Cardiovascular Diabetology, 2010, 9, pp. 21-31. 66. Elliot, W.J., Higher Incidence of Discontinuation of Angiotensin Converting Enzyme Inhibitors Due to Cough in Black Subjects, Clinical Pharmacology and Therapeutics, 1996, 60 (5), pp. 582-588. 67. Tseng, D.S., Kwong, J., Rezvani, F., et al., Angiotensin-converting Enzyme-related Cough Among Chinese-Americans, American Journal of Medicine, 2010, 123 (2), 183, pp. e11-183.e15. 68. Willenheimer, R., van Veldhuisen, D.J., Silke, B., et al., Effect on Survival and Hospitalization of Initiating Treatment for Chronic Heart Failure with Bisoprolol Followed by Enalapril, as Compared with the Opposite Sequence: Results of the Randomized Cardiac Insufficiency Bisoprolol Study (CIBIS) III. Circulation, 2005, 112, pp. 2426-2435.