* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download What public health benefits could be secured by using universal
Survey
Document related concepts
Transcript
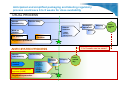
What public health benefits could be secured by using universal labelling & packaging for influenza pandemic vaccines? Workshop on the joint procurement of medical countermeasures 29 April 2015, Luxembourg Sophie Marszalek, Regulatory Affairs Director – Flu Franchise, Sanofi Pasteur Agenda 1 Scope/definition 2 Challenges Anticipated and simplified regulatory process 3 4 5 2 Public health impact of early vaccine availability Vaccines Europe recommendations for the packaging and labeling of pandemic vaccines Scope/definition • A Packaged finished product is composed of o Outer packaging o Primary container (syringe, vial) Outer Containing the vaccine packaging A primary label is stuck o Package Leaflet Label leaflet • These packaging elements are the physical support of vaccinerelated information • The labeling information is compliant with the terms of the marketing authorization and ensure the safe and effective use of the vaccine o Information are made available through the EU QRD template applicable to any drug product in Europe 3 Challenges • During the H1N1 influenza pandemic, companies had difficulties in making available the most up to date package leaflets (PILs) to the patients • Introducing a change in the labeling requires a minimum lead time of 4 weeks from design to actual printing without taking into account the time necessary to include these “new” materials into the production line • Labelling (especially SPC and package leaflet) are subject to changes on a monthly basis 4 Challenges • The inclusion of separate sheets with peel-off stickers in (or with) boxes of multi-dose vials is not considered an ideal approach to minimise risks of misreporting safety issues because: It adds a level of complexity in the production/supply chain and therefore may affect delivery timelines; It may be an additional source of errors and confusion: ¾for example, mistakes/mix-ups are possible at the user-end after vials/syringes are taken out of their original outer packs in preparation for the vaccination (This is probably more likely to happen in large vaccination centers where larger amount of doses are shipped and where the probability of getting boxes from different batches is higher) 5 Anticipated and simplified packaging and labeling regulatory process could save 6 to 8 weeks for dose availability USUAL PROCESS Vaccine manufacturing Vaccine filling Packaging operations Artwork activities Mock up creation Release - Update - Printing - Quality Check Text approval Translation Text proposal Registration procedure License 6 6 to to 8 8 weeks weeks can can be be saved saved ANTICIPATED ANTICIPATED PROCESS PROCESS Vaccine manufacturing Vaccine filling Packaging Packaging operations operations Mock up creation Release Release Common ENGLISH text approval (CORE) License Registration procedure 6 Shipping Shipping Dose Dose availab availab ility ility Dose Dose availab availab ility ility Why early dose availability is crucial for public health in pandemic context? • In a pandemic context, the responsibility of the industry is to develop, produce and deliver as many safe and effective vaccines doses as possible in a timely manner to meet public health expectations and to respond to government's requests • Early availability of the vaccine doses to vaccinate the target population is crucial to: o fight against the disease o have better chances to smoothen/delay the pandemic peak 7 VE recommendations for packaging and labeling of pandemic vaccines • A universal packaging and labeling approach is recommended to reduce the timelines and increase the supply chain flexibility in and outside Europe: “Specific QRD template for pandemic vaccines” with a simplified labelling content for outer packaging, labels and the package leaflet (shortened package leaflet) Early approval with only the essential information in English to ensure the patient safety and secure the instructions for HCPs Up-to-date package leaflet made available on the internet in local languages (e.g. EMA/NRA websites) Vaccines Europe recommendation would be to consider other more appropriate traceability systems (such as a bar code system) 8 How could the universal labeling and packaging look like? • The term of “generic packaging” has often been used Æ “Universal labeling and packaging” would be more appropriate • It would be composed of all the usual packaging elements with essential information in English: (1 set for each manufacturer) Composition, Storage conditions Vaccine (Invented) Name Number of doses Dosage Administration route Batch n° Exp Batch n° Exp.: Vaccine (Invented) Name International Non-Proprietary Name Pharmaceutical form, Dosage Number of doses Administration route Name and address of the Manufacturer Label leaflet No local specificities: -No local registration number -No local bar code 9 Indication Contra-indications Warning and Precautions Special population (e.g. pregnancy, children…) Possible side effects How to use the vaccine A unique contact point for side effects reporting Name and address of the manufacturer Thank You Vaccines Europe / EFPIA Rue du Trône 108 – 1050 Brussels, Belgium Tel +32 2 626 25 55 - www.vaccineseurope.eu