* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download Featured Article
Survey
Document related concepts
Transcript
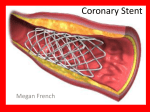
Featured Article www.wfubmc.edu Continuing Medical Education from Clinical Update, Spring 2004 Drug-eluting Stents: The Final Answer to Restenosis? Robert J. Applegate, M.D., FACC, FASCI, FAHA Section of Cardiology, Department of Internal Medicine This Continuing Medical Education activity is sponsored by Wake Forest University School of Medicine. Educational Objectives: 1) Discuss the pathophysiology and clinical manifestations of coronary artery restenosis; 2) Describe the mechanism of benefit of drug eluting stents and outcomes associated with their use in patients with coronary artery disease; 3) Identify patients who would benefit from this therapy. Abstract: Re-narrowing at the angioplasty site, or restenosis, occurs in as many as 50% of patients following percutaneous transluminal coronary angioplasty. Recently, the development of localized drug delivery using drug-eluting stents has markedly reduced the incidence of restenosis. The safety profile of the drug-eluting stents is comparable to that of bare-metal stents in clinical trials reported to date, but only limited data are available about long-term safety of this powerful therapy, which costs about three times more than bare-metal stents. Introduction Andreas Gruentzig performed the first percutaneous transluminal coronary angioplasty (PTCA) in humans in 1977(1) and launched an exciting and innovative therapy for treating patients with coronary artery disease. Despite technological improvements in balloon angioplasty, re-narrowing at the angioplasty site, or restenosis, occurs in up to 30% to 50% of patients following PTCA. The development of coronary stenting reduced the incidence of restenosis after PTCA. However, restenosis of stents themselves occurs in approximately 20% to 40% of patients and remains a significant limitation of this approach. While numerous drug and mechanical interventions have been used in an attempt to minimize or eliminate restenosis, only limited success has been observed with these approaches. Recently, the development of localized drug delivery using stents, or drugeluting stents, has markedly reduced the incidence of restenosis. With this new technology, a new era in the percutaneous management of patients with coronary artery disease has emerged. The phenomenon of restenosis as well as the clinical benefit arising from the use of drug-eluting stents will be reviewed in this article. Pathophysiology of Restenosis Restenosis of the coronary artery following PTCA reflects the interaction of a cascade of molecular and cellular events occurring within the vessel wall. PTCA induces localized injury to the vessel wall, which leads to the release of vasoactive, thrombogenic, and mitogenic factors that result in processes causing re-narrowing at the injured site. Three distinct processes can be identified that contribute to the restenosis process: elastic recoil, arterial remodeling and neointimal hyperplasia. The ability to predict the extent and severity of the restenotic process has been a vexing one. It appears that a combination of patient, lesion, procedural and post-procedural characteristics all contribute to the development of restenosis after coronary angioplasty. Diabetes mellitus and chronic renal insufficiency appear to be the two most dominant clinical factors that predispose patients to restenosis. A host of lesion characteristics, including long lesions, chronic total occlusions, bifurcation disease, small caliber vessels and disease in a saphenous vein graft all are associated with higher rates of restenosis. Finally, a larger post-procedural minimum lumen diameter substantially reduces the risk of restenosis and has led to the concept that “bigger is better”(2). Restenosis Therapies Mechanical devices designed to provide a larger lumen at the completion of the procedure, and pharmacologic therapies designed to inhibit the neointimal proliferation have both been used to reduce restenosis. Debulking procedures, such as directional coronary atherectomy, rotational atherectomy and laser angioplasty, unfortunately have not been associated with clear-cut reduction in the incidence of restenosis. Coronary artery stents(3), however, have reduced the restenosis rate following coronary angioplasty by approximately 50%(4;5). Unfortunately, in-stent restenosis occurs in 20% to 40% of patients. While studies evaluating the effectiveness of pharmacologic agents to reduce restenosis in animal models often were encouraging, use of the same drugs in patients to reduce restenosis has been almost universally disappointing. Several factors likely contribute to the disparity between the results in animal models and humans, but the predominant opinion is that systemic therapy does not provide a high enough concentration of drug at the injured site in the coronary artery to affect cell proliferation. Nonetheless, the apparent ability of a wide variety of chemotherapeutic agents to inhibit the neointimal hyperplastic process in animals paved the way for the concept that if drugs could be administered at a high enough concentration locally in the coronary artery of humans, then the restenotic process may be inhibited by local drug delivery. Drug-Eluting Stents A drug-eluting stent has three elements, all of which have an important effect on vascular injury and restenosis: the metallic stent, a drug-carrier vehicle or coating, and a pharmacologic agent that interferes with local neointimal proliferation(6;7). Current drug-eluting stent systems utilize commercially available stent designs and balloon delivery systems, although newer stent platforms that are designed specifically for drug elution are being evaluated. Stent coatings include biocompatible polymers such as phosphorylcholine, and depot release coatings such as nanoporous ceramic(8). The pharmacologic agents used to interfere with local neointimal formation can be classified as antiproliferative, anti-inflammatory or immune modulating, anti-migratory, antithrombotic and pro-healing agents(6;7). Rapamycin, which is the pharmacologic agent used in the recently released CYPHER stent (Cordis Corporation) interferes with cell proliferation early in the cell cycle (G1 phase arrest) and is considered to be a cytostatic agent. There is currently a great deal of active research into evaluating different and novel pharmacologic agents which also may limit neointimal proliferation. Clinical Outcomes of Drug-eluting Stents Clinical outcomes studies comparing drug-eluting stents to bare-metal stents have shown dramatic reductions in restenosis with drug elution. The first in-man experience with a sirolimus drug-eluting stent (CYPHER, Cordis Corporation), the RAVEL trial and finally the SIRIUS multi-center United States Phase III trial have all demonstrated a substantial reduction in angiographic restenosis (> 50% stenosis)(9-11). In-stent restenosis rates of 0% to 3% and in-segment or vessel restenosis rates of up to only 9% were observed with the drug-eluting stents, compared to 33% in the bare-metal stent arm. Stent thrombosis, major clinical events, and aneurysms at the site of the stent placement were similar in the two treatment groups. These series of clinical trials led the FDA to approve the CYPHER stent for clinical use (April, 2003)(12). Similarly, in the TAXUS II and TAXUS IV randomized clinical trials a substantial reduction in binary restenosis in patients receiving paclitaxel drugeluting stents compared to bare-metal stents was observed at a level comparable to that seen with a sirolimus drug-eluting stent(13;14). Because of these results the FDA has approved the paclitaxel eluting EXPRESS stent (Boston Scientific) for clinical use (Spring 2004). These data indicate that drug-eluting stents can be used resulting in dramatic reduction in restenosis without sacrificing the safety of bare-metal stents. While the results of the studies evaluating the sirolimus and paclitaxel drug-eluting stents have been extremely positive, multiple other studies have been reported demonstrating a seeming lack of efficacy of different drugeluting stents(6;7). These negative studies suggest that the ability of a drug-eluting stent platform to inhibit neointimal proliferation is complex and requires careful and extensive testing before concluding that a drugeluting stent system will be effective in patients. Additionally, it is likely that some biologic agents, no matter how effective when tested in animal models, will not result in an effective drug-eluting stent platform. Since the currently available, or soon to be available, drug-eluting platforms do not entirely eliminate neointimal proliferation, there are opportunities to develop better drug-eluting stent systems. Whether this elusive goal can be achieved remains to be determined. Patients were carefully selected for inclusion in the currently available drug-eluting stent trials. Thus, the benefit of drug-eluting stent use in a broader range of patients not evaluated in clinical trials, including those with acute myocardial infarction, saphenous vein grafts and bifurcation lesions, remains to be determined. Having said that, one of the most significant findings from the SIRIUS trial was that drug-eluting stents were beneficial in a wide range of lesion lengths as well as patient types. Whether the lesion treated was a short segment in a large vessel, or a long segment in a smaller vessel, the relative reduction in restenosis rates with drug-eluting stents were significant. Moreover, diabetic patients also received as significant a benefit from drug-eluting stents as did nondiabetic patients. Recent “real world” clinical outcomes from a consecutive group of patients with acute coronary syndromes treated with drug-eluting stents indicate that clinical restenosis rates and safety profiles are comparable to those achieved in the SIRIUS trial(15). These limited results suggest that the benefits observed in the clinical trials should result in similar outcomes in a broader group of patients. Certainly, more studies and experience will need to be reviewed before it can be unequivocally stated that the ability of drug-eluting stents to reduce neointimal proliferation can be uniformly expected in all patient types and lesion characteristics. Safety Profile of Drug-Eluting Stents While the safety profile of the drug-eluting stents is comparable to that of bare-metal stents in the clinical trials reported to date; only limited data are available about long term-safety of this powerful therapy. One concern in particular merits mention. The incidence of subacute stent thrombosis following the recent clinical release of drug-eluting stents has been of much interest. The FDA recently reported several hundred cases of sub-acute stent thrombosis following use of drug-eluting stents. Because re-endothelialization of drug-eluting stents is believed to occur at a slower rate than that of bare-metal stents, the period of risk for subacute thrombosis may be longer than for conventional stents, and therefore the overall risk may be theoretically higher. Accordingly, the recommendation for the duration of antiplatelet therapy following placement of a drug-eluting stent has been extended from one month to three months. Although the number of cases of subacute stent thrombosis drew considerable attention and concern, when viewed in context of the huge number of drug-eluting stents placed, the actual incidence of subacute stent thrombosis appeared to be well under 1%, which is comparable to or lower than that seen with bare-metal stents. The incidence of stent thrombosis occurring more than three to six months after drug-eluting stent placement remains to be determined, but in small series evaluating the safety of drugeluting stents up to three years, this does not appear to be a significant problem(16). Impact of Drug-Eluting Stents on Health Care Since its release in April of 2003, CYPHER stent usage in the United States has increased dramatically, benefitting patients greatly. However, drug-eluting stents cost approximately three times more than bare-metal stents. The substantial increase in the cost of a drug-eluting stent compared to a bare-metal stent has placed tremendous stress on the healthcare industry, particularly hospitals, which directly bear the brunt of the increased costs. Payment reimbursement for drug-eluting stents has been increased to match the increased cost of a drugeluting stent. However, when more than one drug-eluting stent is placed, with a national standard somewhere between 1.5 and 1.8 stents placed per patient, the full cost of using drug-eluting stents cannot be recovered under current remuneration plans. It is hopeful that as with all new technologies, greater competition in the marketplace will lead to price reductions and minimize the financial pressures on hospitals. Conclusion In conclusion, coronary angioplasty results in lumen enlargement but also localized injury initiating processes resulting in restenosis at the angioplasty site including elastic recoil, negative arterial remodeling and neointimal proliferation. While stents eliminate elastic recoil and negative arterial remodeling, neointimal proliferation within the coronary stent continues to remain a significant clinical problem. Drug-eluting stents, however, substantially reduce neointimal proliferation within a stent without sacrificing the safety profile of conventional stents. Thus, a new and exciting therapy for patients with coronary artery disease has been launched. References 1. Gruentzig AR, Senning A, Siegenthaler WE. Nonoperative dilatation of coronary-artery stenosis. N Engl J Med 1979;301:61-8. 2. Kuntz RE, Safian RD, Carrozza JP, Fishman RF, Mansour M, Baim DS. The importance of acute luminal diameter in determining restenosis after coronary atherectomy or stenting. Circ 1992;86:1827-35. 3. Sigwart U, Kaufmann U, Mirkovitch V, Joffre F, Kappemberger L. Intravascular stents to prevent occlusion and restenosis after transluminal angioplasty. N Engl J Med 1987;316:701-6. 4. Serruys PW, DeJaegere P, Kiemeneij F et al. A comparison of balloon expandable stent implantation with balloon angioplasty in patients with coronary artery disease. N Engl J Med 1994;331:489-95. 5. Fischman DL, Leon MB, Baim D et al. A randomized comparison of coronary stent placement and balloon angioplasty in the treatment of coronary artery disease. N Engl J Med 1994;331:496-501. 6. Sousa JE, Serruys PW, Costa MA. New frontiers in cardiology: Drug-eluting stents: Part I. Circ 2003;107:2274-9. 7. Sousa JE, Serruys PW, Costa MA. New frontiers in cardiology: Drug-eluting stents: Part II. Circ 2003;107:2383-9. 8. van Beusekom HM, Schwartz RS, van der Giessen WJ. Synthetic polymers. Semin Interv Cardiol 1998;3:1458. 9. Morice M-C, Serruys PW, Sousa JE et al. A randomized comparison of a sirolimus-eluting stent with a standard stent for coronary revascularization. N Engl J Med 2002;346:1173-780. 10. Moses JW, Leon MB, Popma JJ et al. Sirolimus-eluting stents versus standard stents in patients with stenosis in a native coronary artery. N Engl J Med 2003;349:1315-23. 11. Sousa JE, Costa MA, Abizaid A et al. Lack of neointimal proliferation after implantation of sirolimus-coated stents in human coronary arteries: A quantitative coronary angiography and three-dimensional intravascular ultrasound study. Circ 2001;103:192-5. 12. US Food and Drug Administration and Center for Devices and Radiological Health. Cypher sirolimus- eluting coronary stent on RAPTOR over-the-wire delivery system or RAPTORRAIL rapid exchange delivery system. US Food and Drug Administration . 2003. 13. Ellis S, Stone GW, Popma JJ et al. The TAXUS IV Study: Final Angiographic Results. [abstr]. Circ 2003;108:IV-532. 14. Colombo A, Drzewiecki J, Banning A et al. Randomized study to assess the effectiveness of slow- and moderate-release polymer-based paclitaxel-eluting stents for coronary artery lesions. Circ 2003;108:788-94. 15. Lemos PA, Lee C, Degertekin M et al. Early outcome after sirolimus-eluting stent implantation in patients with acute coronary syndromes: Insights from the rapamycin-eluting stent evaluated at Rotterdam Cardiology Hospital (RESEARCH) Registry. J Am Coll Cardiol 2003;41:2093-9. 16. Sousa JE, Abizaid A, Abizaid A et al. Late (Three-Year) Follow-Up From the First-in-Man (FIM) Experience After Implantation of Sirolimus-Eluting Stents. [abstr]. Circ 2002;106:II-394. www.wfubmc.edu