* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download Note 7.3 - Translation
Survey
Document related concepts
Transcript
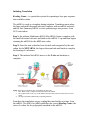
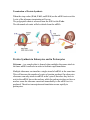
UNIT 3: Molecular Genetics Chapter 7: Genes and Proteins Synthesis pg. 310 - 7.3: Translation: Nucleic Acid to Polypeptide pg. 325 - 331 tRNA Anticodon – is the complementary sequence of base pairs on a tRNA that corresponds to a codon on an mRNA. Aminoacylation – is the process by which a tRNA molecule is bound to its corresponding amino acid. Aminoacyl-tRNA – is a molecule of transfer RNA bound to its associated amino acid. The ribosome can not synthesize a polypeptide chain on its own. The ribosome works in conjunction with transfer RNA to deliver and combine amino acids together, forming peptide bonds, creating a polypeptide chain. Transfer RNA is approximately 70 to 90 nucleotides in length and structurally forms four double helical segments in the shape of a cloverleaf. On the bottom portion of the cloverleaf an Anticodon is located. The Anticodon is complementary to the codon found on the mRNA. The opposite end of the tRNA an amino acid can be attached, which corresponds to the Anticodon. mRNA Codon – 5′AUG3′ tRNA Anticodon – 3′UAC5′ Amino Acid – MET There are 61 codons that code for amino acids, three code for a stop, total 64 codons. When tRNA combines to an amino acid, through the process of Aminoacylation, it creates an Aminoacyl-tRNA. The energy found in the Aminoacyl-tRNA drives the formation of the peptide bond. The ribosome removes the RNA from the amino acids, creating a polypeptide chain. Figure 1: the structure of tRNA: Ribosomes The ribosome is responsible for protein synthesis by translating mRNA into polypeptide chains. The ribosome follows the codon message on the mRNA to produce the appropriate sequence and number of amino acids to be found in the polypeptide chain (primary protein). The ribosome is composed of two different sized parts; large and small ribosomal subunits, made up of a combination of ribosomal RNA and ribosomal protein. Each ribosome has two binding sites that actively bring mRNA and Aminoacyl-tRNAs. The A site (Aminoacyl site) is where the Aminoacyl-tRNA, delivering the next amino acid to be added to the polypeptide chain, binds to the mRNA. The Anticodon aligns itself with the mRNA codon (complementary). The P site (Peptidyl site) is where the tRNA attaches the amino acid to the adjacent amino acid of the polypeptide chain, adding to the growing polypeptide chain. The E site (Exit site) is where the tRNA leaves the ribosome and is released back into the cytoplasm after releasing its amino acid. Figure 3: a ribosome assembles amino acids into a polypeptide chain. A tRNA molecule, with an amino acid bound to it, enters the ribosome on the right (A site). The Anticodon on the tRNA pairs with the codon in the mRNA. Its amino acid will then be added to the growing polypeptide, which is currently attached to the tRNA in the middle of the ribosome (P site). The Process of Translation The process of translation occurs in three stages; Initiation, Elongation, and Termination. When the mRNA binds to a ribosome and the start codon is read (AUG) translation is initiated. AUG codes for the amino acid Methionine. Elongation occurs as more amino acids are attached creating a polypeptide chain. Three nucleotides code for one amino acid, and is known as a reading frame. Termination occurs when the stop codon is read, and translation is stopped. To speed up production of more then one copy of a protein, more then one ribosome may read the same mRNA at the same time, simultaneously translating and making multiple copes of the protein. Initiating Translation Reading Frame – is a particular system for separating a base pair sequence into readable codons. The mRNA is used as a template during initiation. Translation starts when the large and small ribosomal sub-units combine with an mRNA molecule and the first Aminoacyl-tRNA, to start synthesizing a new protein. At an AUG start codon. Step 1: the initiator Methionine-tRNA (Met-tRNA) forms a complex with the small ribosomal sub-unit, and binds to the mRNA 5′ cap and then begins scanning the mRNA for the AUG start codon. Step 2: Once the start codon has been located, and recognized by the anticodon for the MET-tRNA, the larger ribosomal sub-unit binds to complete the forming of a ribosome. Step 3: The initiator Met-tRNA moves to the P-site and initiation is complete. Figure 4: The steps in initiation stage of translation in eukaryotes. 1. Met –tRNA forms a complex with the small ribosomal subunit. 2. The complex binds to the 5′ cp of the mRNA and scans along it until it reaches the AUG start codon. 3. The large ribosomal subunit binds, completing initiation. From here the translation occurs, reading three nucleotides at a time, from the mRNA. The tRNA for AUG establishes the correct Reading Frame, the series of codons for the polypeptide encoded on the mRNA. Elongating the polypeptide Chain Elongation follows four steps; using the A, P, and E sites for growing the polypeptide chain. Step 1: The initiator tRNA attaches to the P-site, the A-site is empty. Step 2: A second tRNA, with complementary anti-codon to the MRNA codon, delivers the amino acid to the A-site of the ribosome. The Met amino acid (AA1) is cleaved from the tRNA in the P-site and forms a peptide bond to second amino acid (AA2) by an enzyme called peptidyl transferase. Step 3: Then moves along one reading frame sequence, with AA1 moving into the E-site and AA2 into the P-site. An appropriate tRNA delivers the next amino acid (AA3) to the A-site. Steps 2 and 3 continue along the mRNA building a polypeptide chain. Step 4: Each empty tRNA (amino acid has been cleaved) is released from the ribosome. Figure 5: The steps in elongation stage of translation. 1. An Aminoacyl-tRNA binds the A site. 2. Peptidyl transferase cleaves the amino acid from the Ps site tRNA and bonds it to the amino acid on the A site tRNA. 3. The ribosome moves along the mRNA to the next codon, thereby bringing the tRNA with the growing polypeptide to the P site and moving the empty tRNA to the E site. 4. After the ribosome has moved over one codon, the empty tRNA in the E site is released ad the cycle is ready to go again. Termination of Protein Synthesis When the stop codon (UAA, UAG, and UGA) on the mRNA arrives at the A-site of the ribosome termination will occur. The polypeptide chain is released from the tRNA at the P-site. The ribosomal sub-units will also detach from the mRNA. Protein Synthesis in Eukaryotes and in Prokaryotes Polysome – is a complex that is formed when multiple ribosomes attach to the same mRNA molecule in order to facilitate rapid translation. Multiple ribosomes can translate a single strand of mRNA at the same time. This will increase the number of copies of protein produced. In eukaryotes ribosomes can only attach to mRNA in the cytosol, therefore they have to wait to the mRNA leaves the nucleus, while the prokaryote does not have a nucleus some the ribosome can attach immediately to the mRNA as it is produced. Therefore transcription and translation occurs rapidly in prokaryotes. Figure 6: Polysomes consist of a series of ribosomes translating the same mRNA See table 1: A comparison of Translation in Prokaryotes and Eukaryotes. Pg. 329 Polypeptide to Protein The primary protein produced is inactive. The protein must be folded into the correct configuration to become active. (primary, secondary, tertiary, and quartary).