* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download Protein Cleavage Due to Pro-oxidative Activity in Some Spices
Survey
Document related concepts
Transcript
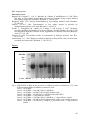
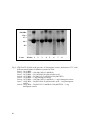
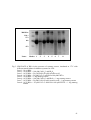
Protein Cleavage Due to Pro-oxidative Activity in Some Spices Sittiwat Lertsiri Department of Biotechnology Faculty of Science, Mahidol University Phayathai, Bangkok 10400 Thailand Kanchana Dumri Department of Chemistry Faculty of Science, Chiang Mai University Chiang Mai 50200 Thailand Keywords: hydroxyl radical, lemongrass, nutmeg, oxidative stress, safflower Abstract To investigate the pro-oxidative activity of nutmeg, safflower, and lemongrass, methanolic extracts of each were incubated with bovine serum albumin (BSA) in the absence or presence of Fe3+/EDTA/H2O2, Fe2+/ascorbate, or Cu2+/H2O2 at 37°C. The protein cleavage by SDS-polyacrylamide gel electrophoresis (SDSPAGE), protein and free amino groups contents were determined. As a result, BSA degradation was observed on SDS-PAGE. This corresponded with decrease in protein concentration and free amino group elevation in the reaction mixture. Such degradation was inhibited in the presence of mannitol, the hydroxyl radical scavenger, indicating that the pro-oxidative activity in these spices caused hydroxyl radical formation. INTRODUCTION Oxidative stress describes a state of damage by reactive oxygen species (ROS), which affects specific molecules or the entire organism. ROS, such as singlet oxygen, superoxide anion, peroxide radical and hydroxyl radical, are generated both intra- and extra-cellularly in all aerobic organisms. They are harmful by their action on vital cellular components including lipids, proteins and nucleic acid. Recent studies showed that various vegetables, fruits, tea, herb, and spices exerted an antioxidant action. The antioxidant capacity of these plant foods is due to the presence of anti-oxidative vitamins (tocopherols, ascorbate), polyphenols, flavonoids, terpenes and various phytochemicals. Some antioxidants such as ascorbic acid, gallic acid and carotenoids, are found to show pro-oxidative activity, depending on the reduction-oxidation potential, concentration and the biological environment. Several flavonoids can also undergo auto-oxidation and generate ROS, such as hydrogen peroxide (Palozza, 1998; Bagnati et al., 1999). This study was conducted to determine the pro-oxidative activity of spices, focusing on the damage of protein due to oxidative stress. The results may lead to better understanding the pro-oxidative activity in the spices. MATERIALS AND METHODS Powdered Spice Samples Nutmeg (Myristica fragrans H.), safflower (Carthamus tinctorius L.), and lemongrass (Cymbopogon citrates L.), were purchased from a local market in Chiang Mai, Thailand. Ten grams of each powdered spices were twice extracted with 75 mL methanol, and filtered through Whatman filter paper No.1, prior to evaporation. Effect of Safflower, Lemongrass, and Nutmeg Extracts on Metal-induced Fragmentation of BSA Effect of spice extracts on metal-induced fragmentation of bovine serum albumin (BSA) was investigated by treating albumin 2 mg/mL under three different conditions: 50 µM FeCl3/3 mM H2O2/100 µM EDTA, 50 µM FeSO4/50 µM ascorbic acid, and 100 µM CuSO4/3 mM H2O2. The systems were in 20 mM phosphate buffer (pH 7.4), incubated at 37°C in the presence or absence of spice extracts as a control in the experiment. Portions were withdrawn from the reaction mixture every 4 h until 24 h and adding butylated Proc. WOCMAP III, Vol.6: Traditional Medicine & Nutraceuticals Eds. U.R. Palaniswamy, L.E. Craker and Z.E. Gardner Acta Hort. 680, ISHS 2005 87 hydroxytoluene methanolic solution to terminate the oxidation. Then, BSA was precipitated from the reaction mixture by adding 20% w/v TCA (final concentration), and centrifuged at 10,000 rpm for 20 min. Fragmentation of albumin was monitored on sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE; Kocha et al., 1997; Winterbourn, 1981). Protein content and free amino groups were measured by Bradford method (Bradford, 1976) and by 2,4,6-trinitrobenzenesulfonic acid (TNBS; Habeeb, 1966), respectively. Effect of Mannitol as Hydroxyl Radical Scavenger on Metal-induced BSA Fragmentation BSA was treated with three different mixtures: 50 µM FeCl3 (0.25 mL)/3 mM H2O2 (0.5 mL)/100 µM EDTA (0.25 mL), 50 µM FeSO4 (0.5 mL)/50 µM ascorbic acid (0.5 mL), and 100 µM CuSO4 (0.5 mL)/3 mM H2O2 (0.5 mL) in the presence of mannitol 10 mM. The reaction mixture was analyzed as mentioned above. RESULTS AND DISCUSSIONS Effect of Safflower, Lemongrass, and Nutmeg Extracts on Metal-induced Fragmentation of BSA The fragmentation of BSA in three different systems were observed on SDSPAGE (Fig. 1, 2 and 3). BSA was fragmented markedly by oxidation reaction in the Cu2+/H2O2 system since the oxidizing power of the Fe2+/ascorbate and Fe3+/ EDTA/H2O2 on the fragmentation of BSA were much weaker than the Cu2+/H2O2 system (Kocha et al., 1997). The pattern of protein bands detected on SDS-PAGE suggested that Fe3+/EDTA/H2O2, Fe2+/ascorbate and Cu2+/H2O2 exerted different oxidation actions in fragmentation of BSA. Moreover, after 12-h incubation, the reaction mixtures containing Cu2+/H2O2 exhibited the fragments of BSA at 33, 40 and 52 kDa (data not shown), while these fragments of BSA were not observed on SDS-PAGE in the other treatments. Protein content examined by Bradford method also decreased with time to 70-80% during the 24-h incubation, compared to the control. These corresponded with the results from the free amino group analysis where the free amino groups increased 20-40% at the end of the incubation. Effect of Mannitol as Hydroxyl Scavenger in Metal-induced BSA Fragmentation Mannitol is one of the most widely used hydroxyl radical scavengers. Therefore, the fragmentation of BSA is inhibited if the hydroxyl radicals are involved in such fragmentation. The data from BSA content determination by Bradford method and free amino group analysis suggested that 10 mM mannitol mostly prevented the fragmentation of BSA under the conditions studied (data not shown). However, the hydroxyl scavenger mannitol did not completely protect the BSA fragmentation in the Cu2+/H2O2 system. This is because BSA could bind tightly to copper ion and led to stronger oxidizing power than the other systems. Mechanisms of Metal-induced BSA Fragmentation The results suggested that the pro-oxidative activity of spice extracts was dependent on dosage and the transition metal. It is well known that metal-induced oxidation catalyzes the fragmentation of proteins. Metal ion can promote hydroxyl radical formation and further bring about protein degradation as observed in BSA and cartilage (Kocha et al., 1997). In the Cu2+/H2O2 system, copper bound to BSA (Cu-BSA) on the specific sites may participate in the Fenton reaction, and hydroxyl radicals would be formed on the BSA molecule surface. This leads to specific pattern of fragmentation. In case of the Fe2+/ascorbate and Fe3+/H2O2/EDTA systems, Fe2+ and Fe3+ weakly bind to BSA molecules without specific binding site. Hence the damage of BSA molecules by hydroxyl radicals occurred randomly on the protein surface. The presence of spice extract would enhance the hydroxyl radical formation, which further promoted 88 BSA fragmentation. Literature Cited Bagnati, M., Perugini, C., Cau, C., Bordone, R., Albano, E. and Bellomo, G. 1999. When and why a water-soluble antioxidants becomes pro-oxidant during copper-induced low-density lipoprotein oxidation. Biochem. J. 340:143-152. Bradford, M.M. 1976. Protein determination by dye-binding method. Anal. Biochem. 72:248-254. Habeeb, A.F.A.S. 1966. Determination of free amino groups in proteins by trinitrobenzenesulfonic acid. Anal. Biochem. 14:328-336. Kocha, T., Yamaguchi, M., Ohtaki, H., Fukuda, T. and Aoyagi, T. 1997. Hydrogen peroxide-mediated degradation of protein: different oxidation modes of copper- and iron-dependent hydroxyl radicals on the degradation of albumin. Biochim. Biophys. Acta 1337:319-326. Palozza, P. 1998. Pro-oxidant actions of carotenoids in biologic systems. Nut. Rev. 56:257-265. Winterbourn, C.C. 1981. Hydroxyl radical production in body fluids: roles of metal ions, ascorbate and superoxide. Biochem. J. 198:125-131. Figures 200 kDa -116------97-----66----- 45----- Lane: Marker 1 2 3 4 5 6 7 8 Fig. 1. SDS-PAGE of BSA in the presence of safflower extract, incubated at 37°C with different metal-induced oxidation systems for 24 h. Lane 1: 10 µg BSA Lane 2: 10 µg BSA + 100 µM CuSO4/3 mM H2O2 Lane 3: 10 µg BSA + 50 µM FeSO4/50 µM Ascorbic acid Lane 4: 10 µg BSA + 50 µM FeCl3/3 mM H2O2/100 µM EDTA Lane 5: 10 µg BSA + 1 mg safflower extract Lane 6: 10 µg BSA + 100 µM CuSO4/3 mM H2O2 + 1 mg safflower extract Lane 7: 10 µg BSA + 50 µM FeSO4/50 µM Ascorbic acid + 1 mg safflower extract Lane 8: 10 µg BSA + 50 µM FeCl3/3 mM H2O2/100 µM EDTA + 1 mg safflower extract 89 200 kDa -116------97-----66----45----- Lane: Marker 1 2 3 4 5 6 7 8 Fig. 2. SDS-PAGE of BSA in the presence of lemongrass extract, incubated at 37°C with different metal-induced oxidation systems for 24 h. Lane 1: 10 µg BSA Lane 2: 10 µg BSA + 100 µM CuSO4/3 mM H2O2 Lane 3: 10 µg BSA + 50 µM FeSO4/50 µM Ascorbic acid Lane 4: 10 µg BSA + 50 µM FeCl3/3 mM H2O2/100 µM EDTA Lane 5: 10 µg BSA + 1 mg lemongrass extract Lane 6: 10 µg BSA + 100 µM CuSO4/3 mM H2O2 + 1 mg lemongrass extract Lane 7: 10 µg BSA + 50 µM FeSO4/50 µM Ascorbic acid + 1 mg lemongrass extract Lane 8: 10 µg BSA + 50 µM FeCl3/3 mM H2O2/100 µM EDTA + 1 mg lemongrass extract 90 200 kDa -116------97-----66----- 45----- Lane: Marker 1 2 3 4 5 6 7 8 Fig. 3. SDS-PAGE of BSA in the presence of nutmeg extract, incubated at 37°C with different metal-induced oxidation systems for 24 h. Lane 1: 10 µg BSA Lane 2: 10 µg BSA + 100 µM CuSO4/3 mM H2O2 Lane 3: 10 µg BSA + 50 µM FeSO4/50 µM Ascorbic acid Lane 4: 10 µg BSA + 50 µM FeCl3/3 mM H2O2/100 µM EDTA Lane 5: 10 µg BSA + 1 mg nutmeg extract Lane 6: 10 µg BSA + 100 µM CuSO4/3 mM H2O2 + 1 mg nutmeg extract Lane 7: 10 µg BSA + 50 µM FeSO4/50 µM Ascorbic acid + 1 mg nutmeg extract Lane 8: 10 µg BSA + 50 µM FeCl3/3 mM H2O2/100 µM EDTA + 1 mg nutmeg extract 91