* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download The Prognostic Value of Left Atrial Peak Reservoir Strain in Acute
Heart failure wikipedia , lookup
Remote ischemic conditioning wikipedia , lookup
Electrocardiography wikipedia , lookup
Hypertrophic cardiomyopathy wikipedia , lookup
Antihypertensive drug wikipedia , lookup
Coronary artery disease wikipedia , lookup
Cardiac contractility modulation wikipedia , lookup
Echocardiography wikipedia , lookup
Cardiac surgery wikipedia , lookup
Lutembacher's syndrome wikipedia , lookup
Arrhythmogenic right ventricular dysplasia wikipedia , lookup
Mitral insufficiency wikipedia , lookup
Jatene procedure wikipedia , lookup
Management of acute coronary syndrome wikipedia , lookup
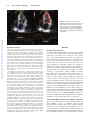
The Prognostic Value of Left Atrial Peak Reservoir Strain in Acute Myocardial Infarction Is Dependent on Left Ventricular Longitudinal Function and Left Atrial Size Mads Ersbøll, MD; Mads J. Andersen, MD; Nana Valeur, MD, PhD; Ulrik Madvig Mogensen, MD; Homa Waziri, MD; Jacob Eifer Møller, MD, PhD, DSci; Christian Hassager, MD, DSci; Peter Søgaard, MD, DSci; Lars Køber, MD, DSci Downloaded from http://circimaging.ahajournals.org/ by guest on June 16, 2017 Background—Peak atrial longitudinal strain (PALS) during the reservoir phase has been proposed as a measure of left atrium function in a range of cardiac conditions, with the potential for added pathophysiological insight and prognostic value. However, no studies have assessed the interrelation of PALS and left ventricular longitudinal strain (global longitudinal strain) in large-scale populations in regard to prognosis. Methods and Results—We prospectively included 843 patients (mean age 62.1±11.8; 74% male) with acute myocardial infarction and measured global longitudinal strain, left atrium volumes, and PALS within 48 hours of admission. PALS was related to a composite outcome of death and heart failure hospitalization. Reduced PALS was associated with hypertension, diabetes mellitus, and Killip class >1 (P<0.05 for all). Reduced PALS was associated with impairment of all measures of left ventricular systolic and diastolic function, and the correlation between global longitudinal strain and PALS was highly significant (P<0.001; r=–0.71). During follow-up (median 23.0 months Q1–Q3, 16.8–26.0), a total of 76 patients (9.0%) reached the composite end point of which 47 patients died (5.6%), and 29 patients were hospitalized for heart failure (3.4%). PALS was significantly associated with outcome (hazard ratio [HR], 0.88; 95% confidence interval [CI], 0.85–0.90; P<0.001); however, no independent effect of PALS (HR, 1.00; 95% CI, 0.94–1.05; P=0.87) was found when adjusting for global longitudinal strain (HR, 1.20; 95% CI, 1.09–1.33; P<0.001), maximum left atrium volume before mitral valve opening (HR, 1.02; 95% CI, 1.01–1.04; P=0.006), and age (HR, 1.06; 95% CI, 1.03–1.08; P<0.001). Conclusions—PALS provides a composite measure of left ventricular longitudinal systolic function and maximum left atrium volume before mitral valve opening, and as such contains no added information when these readily obtained measures are known. (Circ Cardiovasc Imaging. 2013;6:26-33.) Key Words: acute myocardial infarction ◼ atrial strain ◼ echocardiography ◼ longitudinal strain ◼ prognosis L eft atrial dilatation is well recognized as a strong predictor of adverse outcome, after acute myocardial infarction (MI) and in a range of other cardiac pathologies. The left atrium (LA) is directly exposed to left ventricular (LV) cavity pressure during diastole, thus an enlarged LA is a robust marker of increased LV filling pressure in absence of LA volume overload, which provides a causal link between LA dilatation and poor outcome.1 Left atrial function is traditionally described by 3 phases: (1) a reservoir phase during LV systole, (2) conduit phase from the pulmonary vasculature to the LV during early diastole, and (3) active booster pump function in late diastole.2 The phasic LA volumes and the derived volume changes throughout the cardiac cycle have been related to adverse prognosis.3,4 Recently, 2-dimensional speckletracking echocardiography-based analysis of LA deformation during the reservoir phase (peak atrial longitudinal strain [PALS]) has been reported in several studies with encouraging results in hypertension, diabetes mellitus, atrial fibrillation, heart failure (HF), and ST-elevation myocardial infarction (STEMI).5–10 Finally, a recent consensus statement from the European Association of Echocardiography/American Society of Echocardiography (EAE/ASE) has endorsed the promising value of LA-deformation imaging for potentially adding information about preclinical heart disease.11 Clinical Perspective on p 33 Barbier et al12 demonstrated that LA reservoir function is determined by the longitudinal descent of the cardiac base and LA chamber stiffness. The reciprocating changes in LV and LA volumes within the nearly constant total cardiac volume suggest that any measure of LA function will be strongly Received June 26, 2011; accepted November 9, 2012. From The Heart Centre, Department of Cardiology, University Hospital Rigshospitalet, Denmark (M.E., M.J.A., U.M.M., H.W., J.E.M., C.H., L.K.); and Department of Cardiology, University Hospital Gentofte, Denmark (N.V., P.S.). The online-only Data Supplement is available at http://circimaging.ahajournals.org/lookup/suppl/doi:10.1161/CIRCIMAGING.112.978296/-/DC1. Correspondence to Mads Ersbøll, The Heart Centre, Department of Cardiology, University Hospital Rigshospitalet, Denmark. E-mail mads.ersboell@ gmail.com © 2012 American Heart Association, Inc. Circ Cardiovasc Imaging is available at http://circimaging.ahajournals.org 26 DOI: 10.1161/CIRCIMAGING.112.978296 Ersbøll et al Left Atrial Function in Myocardial Infarction 27 influenced by LV longitudinal function. Furthermore, it has been shown that the 4-chambered heart is a nearly constant volume pump within 5±1%, which means that any change in the long-axis dimension of the atrium must be reciprocated by the ventricle and vice versa. Thus, any longitudinal elongation of the atrium must correspond to deformation of the ventricle.13–15 However, none of the studies describing either prognostic or pathophysiological importance of PALS have adjusted for LV longitudinal function that is readily measured by 2-dimensional speckle-tracking echocardiography. Accordingly, the purpose of this study was to assess in patients with MI: (1) the interdependence of LV and LA deformation and (2) the prognostic importance of PALS, when adjusted for both LA size and global LV longitudinal function assessed by global longitudinal strain (GLS). Downloaded from http://circimaging.ahajournals.org/ by guest on June 16, 2017 Methods Study Design and Patient Population Patients with definite MI referred for invasive coronary angiography, either acute because of STEMI or within 1 week of non-STEMI, were prospectively enrolled at 2 tertiary cardiac centers in the Copenhagen region. All patients provided written informed consent prior to transthoracic echocardiographic examination. Exclusion criteria were as follows: aged <18 years, noncardiac disease with a life expectancy of <1 year, or inability to provide written informed consent. Furthermore, patients with atrial fibrillation or paced rhythm during the examination and patients with severe aortic stenosis were excluded from the analyses. Based on hospital records obtained on admission information on diabetes mellitus and hypertension, a history of ischemic heart disease and prior MI was registered. Findings in relation to coronary angiography, including culprit lesion, number of diseased vessels, left main involvement, and type of revascularization (percutanous coronary intervention [PCI], coronary artery bypass grafting [CABG], or no intervention), were registered. Objective signs of HF at presentation or during hospitalization were scored according to the Killip classification scheme. Additional biochemical sampling included creatinin, hemoglobin, and peak troponin during the hospital stay. The study was approved by the Regional Scientific Ethics Committee (reference number H-D-2009–063). Echocardiography Echocardiography was performed within 48 hours of admission to the tertiary center. Echocardiographic cine loops were obtained by recording 3 consecutive heart cycles. All examinations were performed on a Vivid e9 (General Electric, Horten, Norway). Images were obtained at a frame rate of 60 to 90 frames per second and digitally transferred to a remote workstation for offline analysis (Echopac BT 11.1.0, General Electric, Horten, Norway). Three experienced operators performed all patient examinations using a prespecified echocardiographic examination protocol detailing the acquisition of the LA in all 3 apical projections, with specific attention to subsequent strain analysis. All analyses were performed by a single experienced operator (M.E.) blinded to follow-up information. Two-dimensional parasternal images were used to determine LV cavity dimensions and wall thickness. LV mass was calculated from the LV linear dimensions in the parasternal view. Left ventricular ejection fraction (LVEF) was determined using the biplane Simpson method. Wall motion scoring was performed by dividing the LV into 16 segments, and each segment was assigned a score based on myocardial thickening (1, normal or hyperkinesis; 2, hypokinesis; 3, akinesis; and 4, dyskinesis). Wall motion score index was calculated from the average score of all segments. All volumetric and dimensional measurements of the LV were indexed to body surface area when appropriate, in accordance with EAE/ASE recommendations.2 Color Doppler examination of the mitral valve (MV) was performed in the apical window, and if more than trivial mitral regurgitation (MR) was present, it was quantified by calculating the effective regurgitant orifice using the proximal isovelocity surface area method. Effective regurgitant orifice <0.20 cm2 was considered mild, 0.20 to 0.40 cm2 moderate, and >0.40 cm2 severe MR. Pulsed wave Doppler recordings of mitral inflow were performed by placing a 2.5 mm sample volume at the tip of the MV leaflets during diastole. Peak velocity of early (E) and atrial (A) diastolic filling, and MV deceleration time were measured and E/A-ratio calculated. Pulsed wave tissue Doppler Imaging recordings were performed at the lateral and medial mitral annulus using a 2.5 mm sample volume, with measurements of myocardial peak early velocity (e’). The mean E/e’ ratio was calculated from an average of lateral and medial values of e’.16 LV Strain Analysis LV longitudinal function was assessed by GLS using a semiautomatic algorithm (Automated Function Imaging, GE, Horten, Norway). Briefly, 3 points (2 annular and 1 apical) were positioned in each of the 3 apical projections enabling the software to track the myocardium semiautomatically throughout the heart cycle. The region of interest was adjusted to cover the thickness of the myocardium. Aortic valve closure was identified on continuous wave Doppler recording through the aortic valve. Careful inspection of tracking and manual correction, if needed, was performed, and in case of unsatisfactory tracking, the segment would be excluded from the analysis. The Automated Function Imaging algorithm allowed GLS to be calculated for each of the 3 apical projections, if at least 5 out of 6 segments were sufficiently tracked. The algorithm then calculated overall GLS as the average value of all 3 projections. If GLS could only be assessed in 2 of 3 apical projections, we calculated overall GLS as the average of these 2. If GLS could not be assessed in ≥2 of the apical projections, the patient examination was classified as having image quality insufficient for LV strain measurements. LA Volumes and Deformation LA volumes were calculated using the biplane area–length method at 3 distinct points during the cardiac cycle and indexed to body surface area: (1) maximum volume before MV opening (LAmax), (2) minimum volume before MV closing (LAmin), and (3) volume before LA contraction at the onset of the P-wave (LApreA). From these volumes, the following volumetric indices of the LA were calculated: (1) LA reservoir volume change (LAmax–LAmin), (2) LA total emptying fraction 100×([LAmax–LAmin]/LAmax), and (3) Active LA emptying fraction 100×([LApreA–LAmin]/LAmin). LA strain was assessed in the same apical 4C, 2C, and apical long axis view (APLAX) projections used for GLS quantification. The LA endocardial border was traced manually and adjusted to cover the thickness of the LA walls, resulting in strain curves from a total of 18 atrial segments. From the average of all 18 resulting strain curves, we assessed global PALS as the maximum positive strain value during LV systole (Figure 1). Segments were discarded if the tracking algorithm was unable to track the myocardium sufficiently. If more than 2 segments were not tracked properly, the projection was discarded, and if PALS could not be assessed in ≥2 of the apical projections, the patient examination was classified as having image quality insufficient for LA strain measurements. Follow-up and End Point Definition The primary outcome was a composite of death from any cause and hospitalization for HF. Information on all cause mortality was obtained from the Danish Civil Registration System. Information on HF hospitalization was obtained from a systematic review of all hospital admissions after the index MI. Hospitalization from HF was defined as admission because of dyspnea, with objective signs of pulmonary congestion and treatment with intravenous diuretics. Verification of HF hospitalization was performed by an independent reviewer, unknowing of clinical and echocardiographic information relating to the index MI. 28 Circ Cardiovasc Imaging January 2013 Figure 1. Example of 2-dimensional speckle-tracking of the left atrium (LA; top left) and the LV (top right). The resulting strain curves for the LA (lower left) and LV (lower right) are shown with markings corresponding to peak atrial longitudinal strain (PALS) and peak global longitudinal strain (GLS). Downloaded from http://circimaging.ahajournals.org/ by guest on June 16, 2017 Statistical Analysis All data are reported as mean±SD or median (first and third quartile, Q1–Q3). Baseline clinical and echocardiographic data were analyzed according to GLS quartiles, using Cochran-Armitage trend test for categorical variables and ANOVA for continuous variables. All tests were 2-sided, and statistical significance was defined as P<0.05. Interobserver and intraobserver reproducibility of PALS and GLS was assessed in 20 randomly selected patients, with calculation of bias (mean difference) and limits of agreement (±1.96 SD). The relationship between PALS and GLS were examined by correlation analysis. To explore the bivariate impact of LA volume and GLS on PALS, we performed multiple linear regression analysis with PALS as the dependent variable. Furthermore, the proportion of variance in PALS explained by GLS and LAmax, respectively, was calculated by partial correlation analysis. The ability of measures of LA volumes, LA deformation indices, and GLS for prediction of the composite end point was examined in univariate Cox proportional hazard models. To examine the independent and added prognostic value of PALS, we first created a clinical Cox model consisting of age, history of diabetes mellitus, Killip class >1, and type of infarction (STEMI/non-STEMI). Into this Cox model was then added (1) LVEF and LAmax, (2) GLS, and (3) PALS, with calculation of –2 log likelihood, Akaike Information Criterion, and concordance index at each step. The incremental improvement in model performance was assessed from these parameters. Furthermore, we added PALS to a Cox model consisting of only age, GLS, and LAmax to explore the independent value of PALS (1) without the constraints of a relatively limited number of events and (2) motivated by the findings in the regression analyses of PALS, GLS, and LAmax. Finally, each of the derived LA volumes was modeled in separate Cox models adjusted for GLS, age, and LAmax as forced entry covariates. Assumptions of linearity and proportionality were assessed with cumulated Martingale- and Schoenfeld residuals, respectively. Both GLS and PALS violated the proportionality assumption, thus a constant hazard ratio (HR) was calculated for the time interval up until the first year, and a constant HR at another level after the first year. The Cox model, including PALS, GLS, age, and LAmax, was tested in 1000 bootstrap samples to assess the potential effect of PALS in randomly regenerated data. All statistical analyses were performed using R software (R Development Core Team (2012). R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. ISBN 3-900051-07-0, URL http://www.R-project.org/). Results Baseline Characteristics A total of 1110 patients with MI were prospectively included, 53 patients were excluded because of atrial fibrillation (n=40), ventricular paced rhythm (n=5), and severe aortic stenosis (n=8). Out of the remaining 1057 patients, 51 patients had images insufficient for GLS calculation (5%) and 163 (15%) did not have sufficient tracking of the LA walls. Thus, 843 patients (mean age 62.1±11.8; 74% male) out of the total study population of 1110 (76%) patients were eligible for analyses in the present study. The baseline characteristics of the patients according to quartiles of PALS are listed in Table 1. Reduced PALS was associated with increasing age, higher prevalence of hypertension, diabetes mellitus, previously known HF, and in-hospital heart failure (Killip class >1). Echocardiographic characteristics according to quartiles of PALS are shown in Table 2. Reduced PALS was significantly associated with all echocardiographic measures of reduced systolic and diastolic function, as well as decreasing values of LA functional parameters. The 163 patients without obtainable PALS were not significantly different compared with the 843 patients in terms of LVEF, GLS, LAmax, LAmin, or E/e’ ratio. Furthermore, no clinical differences were found in regard to age, diabetes mellitus, known HF, prior MI, or proportion of STEMI; however, hypertension was more prevalent among the excluded patients (56% versus 45%; P<0.001) and BMI was higher (27.7 versus 26.5; P<0.001). Intraobserver and interobserver variability for PALS was –0.71±2.20% and 0.31±3.8%, respectively. Interrelation Between PALS, LA Volumes, and GLS There was a significant linear relation between PALS and GLS (P<0.001, r=–0.71). A curvilinear relation existed between PALS and LAmax with greater variation of PALS in the lower ranges of LAmax, whereas higher LAmax was associated with a more definite reduction in PALS (Figure 2A and 2B). Multiple regression analysis of PALS demonstrated that both GLS and LAmax were independently associated Ersbøll et al Left Atrial Function in Myocardial Infarction 29 Table 1. Baseline Clinical Characteristics According to Quartiles of PALS PALS (%) >32.3 n=211 27.3–32.3 n=211 22.5–27.3 n=211 <22.5 n=210 P value Age, y 56.8±11.2 60.1±10.7 63.1±11.2 68.6±10.7 <0.001 Male sex 155 (74.2) 158 (75.9) 160 (77.3) 139 (66.5) 0.11 BMI, kg/m2 25.9±3.8 26.9±4.2 26.8±3.7 26.4±4.4 <0.05 <0.001 Characteristic Medical history Hypertension 60 (28.7) 88 (42.3) 104 (50.2) 118 (56.5) Previous MI 24 (11.5) 25 (12.0) 20 (9.7) 33 (15.8) 0.25 Diabetes mellitus 15 (7.2) 27 (13.0) 24 (11.6) 41 (19.6) <0.001 150 (71.8) 143 (68.8) 152 (73.4) 133 (63.6) 0.21 3 (1.4) 5 (2.4) 7 (3.4) 26 (12.4) <0.001 Smoking Heart failure eGFR, mL/min per 1.73 m2 Downloaded from http://circimaging.ahajournals.org/ by guest on June 16, 2017 82.9±12.0 78.3±15.0 73.6±18.9 <0.001 Killip class >1 83.2±9.5 7 (3.4) 7 (3.4) 32 (15.5) 74 (35.4) <0.001 Heart rate, beats/min 69±11.6 69±10.6 73±12.7 79±14.0 <0.001 Systolic 130±17.2 134±19.7 128±21.7 126±23.1 <0.05 Diastolic 79±11.0 81±10.9 79±12.3 79±14.2 0.34 0.07 Blood pressure, mm Hg Infarct classification 77 (36.5) 66 (31.3) 52 (24.6) 65 (31.0) 134 (63.5) 145 (68.7) 159 (75.4) 145 (69.0) 0.14 LAD involvement 68 (32.5) 73 (35.1) 93 (44.9) 104 (49.8) <0.001 Multivessel disease 23 (11.0) 24 (11.5) 28 (13.5) 62 (29.7) <0.001 Non-STEMI STEMI Intervention Primary PCI 125 (59.2) 138 (65.4) 148 (70.1) 122 (58.1) 0.97 Subacute PCI 37 (17.5) 34 (16.1) 31 (14.7) 36 (17.1) 0.88 No PCI 49 (23.3) 39 (18.5) 32 (15.2) 52 (24.8) 0.86 Additional CABG 14 (6.7) 13 (6.3) 19 (9.2) 26 (12.4) 0.01 BMI indicates body mass index; CABG, coronary artery bypass grafting; eGFR, estimated glomerular filtration rate; LAD, left anterior descending artery; PALS, peak atrial longitudinal strain; PCI, percutaneous coronary intervention; and STEMI, ST-elevation myocardial infarction. with PALS. However, the partial correlation of PALS and LAmax after removal of the effect of GLS was lower when compared with that between PALS and GLS after the removal of LAmax (–0.33 versus –0.71), confirmed by a bivariate distribution plot of PALS as a function of GLS and LAmax quartiles (Figure 3). Relation Between PALS, LA Volumes, GLS, and Outcome During follow-up (median 23.0 months Q1–Q3, 16.8–26.0), a total of 76 patients (9.0%) reached the combined end point, of which 47 patients died (5.6%) and 29 patients were hospitalized for HF (3.4%). Global PALS was significantly associated with outcome (HR, 0.88; 95% confidence interval [CI], 0.85–0.90; P<0.001) in univariate analysis. Both addition of LVEF and LAmax to the clinical model and subsequent addition of GLS improved overall model fit as assessed by the decrease in –2 log likelihood and Akaike Information Criterion. Addition of PALS did not improve model performance further and, in fact, worsened the Akaike Information Criterion because of the built-in penalization of overfitting. Results of the multivariable stepwise modeling are given in Table 3. Adjustment for age, GLS, and LAmax removed the effect of PALS (HR, 0.97; 95% CI, 0.92–1.03; P=0.44) without the time-varying effect. When modeling the effect of GLS and PALS with constant HRs up until the first year, GLS continued to be independently prognostic (first year HR, 1.20; 95% CI, 1.09–1.33; P<0.001 and after first year HR, 1.26; 95% CI, 1.13–1.40; P<0.001), whereas PALS remained nonsignificant (HR first year, 1.00; 95% CI, 0.94–1.05; P=0.87). Age (HR, 1.06; 95% CI, 1.03– 1.08; P<0.001) and LAmax (HR, 1.02; 95% CI, 1.01–1.04; P=0.006) remained independently associated with outcome. Because of a high degree of correlation, we assessed LAmin and LAmax in 2 different models, all other covariates being unchanged, and overall model performance was similar (both C-index=0.81). No significant interactions between GLS and LAmax or LAmin could be detected in the Cox models. Finally, we assessed the added contribution of the derived LA volumes previously defined for the prediction of the composite end point. When GLS, age, and LAmax were in the models, LA reservoir volume change (HR, 0.97; 95% CI, 0.91–1.02; P=0.25), LA total emptying fraction (HR, 0.99; 95% CI, 0.97–1.01; P=0.22), and active LA emptying fraction (HR, 1.00; 95% CI 0.99–1.02, P=0.40) did not contain independent prognostic information, whereas GLS, age, and LAmax were independently prognostic. 30 Circ Cardiovasc Imaging January 2013 Table 2. Baseline Echocardiographic Characteristics According to Quartiles of PALS PALS (%) Characteristic >32.3 n=211 27.3–32.3 n=211 22.5–27.3 n=211 <22.5 n=210 P value LV systolic function LV EDV, mL 84±23 87±25 89±26 100±37 <0.001 LV ESV, mL 37±14 42±17 45±19 60±31 <0.001 LVEF, % 56±7 53±8 50±9 42±12 <0.001 WMSI 1.2±0.2 1.3±0.2 1.5±0,3 1.7±0.3 <0.001 LVMI, g/m2 81±18 86±21 96±25 105±30 <0.001 –16.8±2.4 –15.0±2.6 –13.0±2.5 –10.3±3.1 <0.001 GLS, % Left atrial volumes (mL/m2) Downloaded from http://circimaging.ahajournals.org/ by guest on June 16, 2017 LAmax 32±9 33±10 34±10 40±12 <0.001 LAmin 15±5 17±6 18±6 25±10 <0.001 LApreA 22±7 24±7 26±8 33±12 <0.001 LA reservoir volume change 17±6 16±6 16±5 15±6 0.02 LA total emptying fraction 52±8 48±14 46±8 38±10 <0.001 LA active emptying fraction 48±24 45±27 41±22 32±21 <0.001 LV diastolic function E/e’ ratio 8.5±2.2 9.4±2.8 10.9±3.8 14.5±6.4 <0.001 E/A ratio 1.1±0.3 1.0±0.3 1.0±0.3 1.3±0.7 <0.001 MV Dec. time, ms 196±45 194±49 188±50 166±55 <0.001 0 (0) 4 (2) 5 (2) 19 (9) <0.001 2.4 (0.4) 2.3 (0.4) 2.1 (0.4) 1.9 (0.4) <0.001 MR moderate-to-severe TAPSE EDV indicates end-diastolic volume; EF, ejection fraction; ESV, end-systolic volume; GLS, global longitudinal strain; LA, left atrium; LV, left ventricle; LVMI, left ventricular mass index; MV, mitral valve; PALS, peak atrial longitudinal strain; TAPSE, tricuspid annular plane systolic excursion; and WMSI, wall motion score index. Discussion The major findings of the present study were as follows: (1) Reduced PALS is significantly related to the burden of comorbidities, progressively deteriorating indices of LV systolic and diastolic function, and predicted adverse outcome; (2) PALS is closely associated with GLS and to a lesser extent LA dilatation per se; (3) PALS does not add further information in regard to adverse outcome, when readily obtainable indices of LV longitudinal systolic function and diastolic indices are known; and (4) GLS and LA dilatation seem to be the major drivers of apparent Figure 2. A, (red) Inverse relationship between peak atrial longitudinal strain (PALS) and global longitudinal strain (GLS). B, (blue) inverse relationship between PALS and left atrial maximum volume (LAmax). Ersbøll et al Left Atrial Function in Myocardial Infarction 31 Figure 3. Bivariate distribution of peak atrial longitudinal strain (PALS) values as a function of global longitudinal strain (GLS) and left atrial maximum volume (LAmax) quartiles with axes showing the direction of impaired GLS and dilated left atrium (LA) and the resulting reductions in PALS. Worsening GLS confers larger reduction in PALS given constant LAmax, whereas progressively dilated LA given a constant GLS reduces PALS by a smaller magnitude. Downloaded from http://circimaging.ahajournals.org/ by guest on June 16, 2017 LA functional deterioration, questioning the independent and added value of assessing LA deformation in patients with MI. LA Function in Relation to GLS The LA reservoir phase has been shown to depend initially on LA-relaxation properties after the preceding LA contraction, and later the downward motion of the mitral plane driven by LV longitudinal shortening but modulated by LA compliance.12 This study also demonstrated that right ventricular (RV) function was not a determinant of LA reservoir function. We find that tricuspid annular plane systolic excursion is progressively diminished with reduced PALS; however, this would also be confounded by impaired GLS, as more extensive LV myocardial damage after the MI would inherently increase the likelihood of simultaneous RV involvement. Whether RV strain utilizing deformation analysis would impact on PALS, independently of GLS and LAmax, should be explored in future studies. Reduced PALS was associated with moderateto-severe MR that has been demonstrated previously7; however, both LA dilatation through increased volume overloading and impaired GLS18 are also associated with MR. Only 28 patients (3.3%) had moderate-to-severe MR and were included in the analyses as PALS, as well as GLS and LAmax, was expected to be related to the MR. Previous studies have shown that PALS, as a measure of LA reservoir function, exhibits distinct abnormalities in a number of conditions, including hypertension, diabetes mellitus, STEMI, cardiomyopathies, and HF with preserved LVEF.6,9,10,19 Based on this, PALS has been proposed as a measure of LA intrinsic functional properties reflecting earlier stages of Table 3. Stepwise Multivariable Cox Regression Modeling Model 1 Clinical Model 2 Clinical+LVEF+LA β P Age 1.07 Diabetes 1.23 Killip class >1 Type of infarction (STEMI/nSTEMI) Model 3 Clinical+LVEF+LA+GLS Model 3 Clinical+LVEF+LA+GLS+PALS β P β P β <0.0001 1.06 <0.0001 1.06 <0.0001 1.06 <0.0001 0.47 1.14 0.64 1.09 0.77 1.09 0.76 3.44 <0.0001 1.79 0.03 1.47 0.17 1.47 0.16 1.00 0.99 1.01 0.96 0.96 0.86 0.95 0.83 LVEF 0.94 <0.0001 0.96 0.0005 0.95 0.0005 LAmax 1.02 0.048 1.02 0.041 1.02 0.04 GLS (first year) 1.18 0.03 1.18 0.03 GLS (later than first year)* 0.93 0.34 0.93 0.35 PALS (first year) 1.00 0.86 PALS (later than first year)* 0.96 0.32 Covariate –2 log likelihood 927 882 876 876 AIC 934 894 890 893 Concordance index 0.79 0.83 0.83 0.83 AIC indicates Akaike Information Criterion; GLS, global longitudinal strain; LAmax, left atrial maximum volume; LVEF, left ventricular ejection fraction; PALS, peak atrial longitudinal strain; and STEMI, ST elevation myocardial infarction. *Conditioned on having survived the first year without event. P 32 Circ Cardiovasc Imaging January 2013 diseases processes.20 However, in both hypertension and diabetes mellitus, early impairment of LV longitudinal function can be detected by GLS before the onset of overt LV systolic dysfunction,21,22 patients with STEMI exhibit marked impairment in GLS,23 and HF with preserved LVEF has been associated with impaired GLS.24 The present study demonstrates in the largest population so far, with simultaneous measurements of GLS and PALS, that these measures are collinear in nature and that PALS is a reflection of GLS and LA dilatation. PALS and GLS in Relation to Prognosis Downloaded from http://circimaging.ahajournals.org/ by guest on June 16, 2017 The prognostic value of PALS was significant for the prediction of the combined end point death or HF hospitalization during follow-up. Multivariable adjustment eliminated the effect of PALS and overall model fit was not improved by the addition of this parameter, when clinical information, LVEF, LAmax, and GLS were in the model. Furthermore, when adjusted only for GLS, LAmax, and age, there was still no significant effect of PALS, which indicates that it contains no independent prognostic value, and the adjusted HR of PALS approaching 1.00 indicates that the results are not because of overfitting or low power. Bootstrap validation found a significant independent effect of PALS in only 10% of the 1000 samples, which further argues against an independent effect of PALS. These findings are consistent with the results of the linear regression analyses and together substantiate the argument that PALS is merely a composite measure of GLS and LA size. Furthermore, none of the derived LA volumes emerged as independent predictors when GLS, LAmax, and age were in the model, despite a relatively large sample size of 843 patients with 76 events. Thus far, only 1 large-scale study (n=320) has examined the effect of PALS in relation to prognosis, reporting a univariate HR of 0.93 for a composite outcome of death, HF, and new MI,9 which is comparable with our results. However, no adjustment for GLS was performed, although GLS is known to be reduced in MI23 and associated with prognosis.25 Although experimental data have demonstrated that reservoir function also depends on LA compliance and LA relaxation, this study suggests that the currently available speckle-tracking techniques do not capture information beyond that obtained with GLS and LAmax. The fundamental physiology of the near-constant volume of 4-chambered heart predict that elongation of the LA during LV systole, as assessed by PALS, must be reciprocated by the longitudinal shortening of the LV (GLS).13–15 This implies that from a physiological viewpoint the value of PALS as a marker of early disease or poor outcome is heavily confounded by GLS. In this study, we provide the empirical evidence in a large population of patients with MI that PALS has no independent role and is merely a reflection of LV longitudinal function. It is conceivable that an isolated atrial myopathy without involvement of LV longitudinal function could be well characterized with PALS; however, apart from true lone atrial fibrillation and stiff LA syndrome,26 GLS will be an important confounder in this regard. As LAmax is readily measured from standard projections and with GLS becoming increasingly automated, it seems counterintuitive given the present study findings to elaborately quantify PALS. Limitations A significant proportion of patients did not have images suitable for LA strain assessment and the measurements are challenging. The algorithm for strain quantification was designed for LV quantification, and as such not developed for LA strain analysis. PALS was calculated from the average value of all 6 segments in each of the 3 apical projections, including the LA roof region. Some studies have included only mid-LA segments in all 3 apical projections,9 whereas others have included all 3 projections, but omitted the LA region bordering the ascending aorta in the apical long-axis view and used the p-wave as time reference for tracking.11 However, no definite consensus exists as to how many LA segments should be included in the analysis. Finally, our results could be because of low power preventing the detection of a true prognostic effect of PALS; however, only 10% of the randomly regenerated bootstrap samples resulted in an independent effect of PALS, which validates our findings internally. Conclusions The magnitude of PALS as assessed with 2-dimensional speckle tracking in patients with acute MI is dependent on GLS and LA size. Measurement of PALS confers no independent prognostic value, when these readily obtainable echocardiographic parameters are known. The added value of LA reservoir function in patients with impaired LV longitudinal function is questionable. Sources of Funding The authors would like to thank the following for providing financial assistance with echocardiographic equipment and analytical software: Fondation Juchum, Switzerland; Beckett Fonden, Denmark; Toyota Fonden, Denmark. Disclosures None. References 1. Moller JE, Hillis GS, Oh JK, Seward JB, Reeder GS, Wright RS, Park SW, Bailey KR, Pellikka PA. Left atrial volume: a powerful predictor of survival after acute myocardial infarction. Circulation. 2003;107:2207–2212. 2. Lang RM, Bierig M, Devereux RB, Flachskampf FA, Foster E, Pellikka PA, Picard MH, Roman MJ, Seward J, Shanewise JS, Solomon SD, Spencer KT, Sutton MS, Stewart WJ; Chamber Quantification Writing Group; American Society of Echocardiography’s Guidelines and Standards Committee; European Association of Echocardiography. Recommendations for chamber quantification: a report from the American Society of Echocardiography’s Guidelines and Standards Committee and the Chamber Quantification Writing Group, developed in conjunction with the European Association of Echocardiography, a branch of the European Society of Cardiology. J Am Soc Echocardiogr. 2005;18:1440–1463. 3. Kühl JT, Møller JE, Kristensen TS, Kelbæk H, Kofoed KF. Left atrial function and mortality in patients with NSTEMI an MDCT study. JACC Cardiovasc Imaging. 2011;4:1080–1087. 4. Gupta S, Matulevicius SA, Ayers CR, Berry JD, Patel PC, Markham DW, Levine BD, Chin KM, de Lemos JA, Peshock RM, Drazner MH. Left atrial structure and function and clinical outcomes in the general population. Eur Heart J. 2012;epub:July 10, 2012. 5. Inaba Y, Yuda S, Kobayashi N, Hashimoto A, Uno K, Nakata T, Tsuchihashi K, Miura T, Ura N, Shimamoto K. Strain rate imaging for noninvasive functional quantification of the left atrium: comparative studies in controls and patients with atrial fibrillation. J Am Soc Echocardiogr. 2005;18:729–736. Ersbøll et al Left Atrial Function in Myocardial Infarction 33 Downloaded from http://circimaging.ahajournals.org/ by guest on June 16, 2017 6. Muranaka A, Yuda S, Tsuchihashi K, Hashimoto A, Nakata T, Miura T, Tsuzuki M, Wakabayashi C, Watanabe N, Shimamoto K. Quantitative assessment of left ventricular and left atrial functions by strain rate imaging in diabetic patients with and without hypertension. Echocardiography. 2009;26:262–271. 7. Kuppahally SS, Akoum N, Badger TJ, Burgon NS, Haslam T, Kholmovski E, Macleod R, McGann C, Marrouche NF. Echocardiographic left atrial reverse remodeling after catheter ablation of atrial fibrillation is predicted by preablation delayed enhancement of left atrium by magnetic resonance imaging. Am Heart J. 2010;160:877–884. 8. Cameli M, Lisi M, Focardi M, Reccia R, Natali BM, Sparla S, Mondillo S. Left atrial deformation analysis by speckle tracking echocardiography for prediction of cardiovascular outcomes. Am J Cardiol. 2012;110:264–269. 9. Antoni ML, ten Brinke EA, Atary JZ, Marsan NA, Holman ER, Schalij MJ, Bax JJ, Delgado V. Left atrial strain is related to adverse events in patients after acute myocardial infarction treated with primary percutaneous coronary intervention. Heart. 2011;97:1332–1337. 10. Roşca M, Popescu BA, Beladan CC, Călin A, Muraru D, Popa EC, Lancellotti P, Enache R, Coman IM, Jurcuţ R, Ghionea M, Ginghină C. Left atrial dysfunction as a correlate of heart failure symptoms in hypertrophic cardiomyopathy. J Am Soc Echocardiogr. 2010;23:1090–1098. 11.Mor-Avi V, Lang RM, Badano LP, Belohlavek M, Cardim NM, Derumeaux G, Galderisi M, Marwick T, Nagueh SF, Sengupta PP, Sicari R, Smiseth OA, Smulevitz B, Takeuchi M, Thomas JD, Vannan M, Voigt JU, Zamorano JL. Current and evolving echocardiographic techniques for the quantitative evaluation of cardiac mechanics: ASE/EAE consensus statement on methodology and indications endorsed by the Japanese Society of Echocardiography. J Am Soc Echocardiogr. 2011;24:277–313. 12. Barbier P, Solomon SB, Schiller NB, Glantz SA. Left atrial relaxation and left ventricular systolic function determine left atrial reservoir function. Circulation. 1999;100:427–436. 13. Appleton CP, Kovács SJ. The role of left atrial function in diastolic heart failure. Circ Cardiovasc Imaging. 2009;2:6–9. 14. Bowman AW, Kovács SJ. Assessment and consequences of the constantvolume attribute of the four-chambered heart. Am J Physiol Heart Circ Physiol. 2003;285:H2027–H2033. 15.Bowman AW, Kovács SJ. Left atrial conduit volume is generated by deviation from the constant-volume state of the left heart: a combined MRI-echocardiographic study. Am J Physiol Heart Circ Physiol. 2004;286:H2416–H2424. 16.Nagueh SF, Appleton CP, Gillebert TC, Marino PN, Oh JK, Smis eth OA, Waggoner AD, Flachskampf FA, Pellikka PA, Evangelisa A. Recommendations for the evaluation of left ventricular diastolic function by echocardiography. Eur J Echocardiogr. 2009;10:165–193. 17. Moustafa SE, Alharthi M, Kansal M, Deng Y, Chandrasekaran K, Mookadam F. Global left atrial dysfunction and regional heterogeneity in primary chronic mitral insufficiency. Eur J Echocardiogr. 2011;12: 384–393. 18. Mascle S, Schnell F, Thebault C, Corbineau H, Laurent M, Hamonic S, Veillard D, Mabo P, Leguerrier A, Donal E. Predictive value of global longitudinal strain in a surgical population of organic mitral regurgitation. J Am Soc Echocardiogr. 2012;25:766–772. 19. Kurt M, Wang J, Torre-Amione G, Nagueh SF. Left atrial function in diastolic heart failure. Circ Cardiovasc Imaging. 2009;2:10–15. 20. Shah AM, Solomon SD. Myocardial deformation imaging: current status and future directions. Circulation. 2012;125:e244–e248. 21. Dalen H, Thorstensen A, Romundstad PR, Aase SA, Stoylen A, Vatten LJ. Cardiovascular risk factors and systolic and diastolic cardiac function: a tissue Doppler and speckle tracking echocardiographic study. J Am Soc Echocardiogr. 2011;24:322–32.e6. 22. Nakai H, Takeuchi M, Nishikage T, Lang RM, Otsuji Y. Subclinical left ventricular dysfunction in asymptomatic diabetic patients assessed by two-dimensional speckle tracking echocardiography: correlation with diabetic duration. Eur J Echocardiogr. 2009;10:926–932. 23. Gjesdal O, Helle-Valle T, Hopp E, Lunde K, Vartdal T, Aakhus S, Smith HJ, Ihlen H, Edvardsen T. Noninvasive separation of large, medium, and small myocardial infarcts in survivors of reperfused ST-elevation myocardial infarction: a comprehensive tissue Doppler and speckle-tracking echocardiography study. Circ Cardiovasc Imaging. 2008;1:189–96, 2 p following 196. 24. Wang J, Khoury DS, Yue Y, Torre-Amione G, Nagueh SF. Preserved left ventricular twist and circumferential deformation, but depressed longitudinal and radial deformation in patients with diastolic heart failure. Eur Heart J. 2008;29:1283–1289. 25. Antoni ML, Mollema SA, Delgado V, Atary JZ, Borleffs CJ, Boersma E, Holman ER, van der Wall EE, Schalij MJ, Bax JJ. Prognostic importance of strain and strain rate after acute myocardial infarction. Eur Heart J. 2010;31:1640–1647. 26. Gibson DN, Di Biase L, Mohanty P, Patel JD, Bai R, Sanchez J, Burkhardt JD, Heywood JT, Johnson AD, Rubenson DS, Horton R, Gallinghouse GJ, Beheiry S, Curtis GP, Cohen DN, Lee MY, Smith MR, Gopinath D, Lewis WR, Natale A. Stiff left atrial syndrome after catheter ablation for atrial fibrillation: clinical characterization, prevalence, and predictors. Heart Rhythm. 2011;8:1364–1371. Clinical Perspective Left atrium (LA) dilatation in the aftermath of acute myocardial infarction (MI) is a marker of long-standing elevated left ventricular filling pressure and is associated with an adverse prognosis. Recently, attention has been directed toward a functional evaluation of the LA using 2-dimensional speckle-tracking, with several studies reporting a better discriminative and prognostic performance of these parameters than the more static LA maximum volume. A limitation to these studies, however, is the lack of consideration of the fundamental physiology of the near-constant volume pump, which states that any elongation of the atrium must be reciprocated by shortening of the ventricle (LV). Thus, the studies reporting peak atrial longitudinal strain (PALS) had potential confounding by LV long-axis function. This study correlated PALS with global LV longitudinal strain (GLS) in a large population of patients with MI and assessed the prognostic impact of LA function in relation to GLS and LA maximum volume. We found that PALS and GLS were highly collinear, and that the prognostic information of PALS is dependent on its interrelation with GLS and LA maximum volume. These findings are predictable from the basic physiology of the near-constant volume pump heart. The results of this study provide empirical evidence to support the concept that LA function cannot be considered an independent entity or independent prognostic marker, when LV long-axis function and LA maximum volume are known. The Prognostic Value of Left Atrial Peak Reservoir Strain in Acute Myocardial Infarction Is Dependent on Left Ventricular Longitudinal Function and Left Atrial Size Mads Ersbøll, Mads J. Andersen, Nana Valeur, Ulrik Madvig Mogensen, Homa Waziri, Jacob Eifer Møller, Christian Hassager, Peter Søgaard and Lars Køber Downloaded from http://circimaging.ahajournals.org/ by guest on June 16, 2017 Circ Cardiovasc Imaging. 2013;6:26-33; originally published online November 27, 2012; doi: 10.1161/CIRCIMAGING.112.978296 Circulation: Cardiovascular Imaging is published by the American Heart Association, 7272 Greenville Avenue, Dallas, TX 75231 Copyright © 2012 American Heart Association, Inc. All rights reserved. Print ISSN: 1941-9651. Online ISSN: 1942-0080 The online version of this article, along with updated information and services, is located on the World Wide Web at: http://circimaging.ahajournals.org/content/6/1/26 Permissions: Requests for permissions to reproduce figures, tables, or portions of articles originally published in Circulation: Cardiovascular Imaging can be obtained via RightsLink, a service of the Copyright Clearance Center, not the Editorial Office. Once the online version of the published article for which permission is being requested is located, click Request Permissions in the middle column of the Web page under Services. Further information about this process is available in the Permissions and Rights Question and Answer document. Reprints: Information about reprints can be found online at: http://www.lww.com/reprints Subscriptions: Information about subscribing to Circulation: Cardiovascular Imaging is online at: http://circimaging.ahajournals.org//subscriptions/