* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download Aromatic Compounds Early in the history of organic chemistry (late
Survey
Document related concepts
Transcript
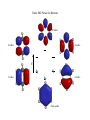
Aromatic Compounds Early in the history of organic chemistry (late 18th, early 19th century) chemists discovered a class of compounds which were unusually stable A number of these compounds had a distinct odor Hence these compounds were called “aromatic” Today the term aromatic is used regardless of the odor of the compound Some “aromatic” compounds have little to no odor Benzene The parent aromatic compound was discovered to have a molecular formula of C6H6 This 1:1 ratio of carbon to hydrogen is extremely low compared to other known compounds It was also quickly discovered that these aromatic compounds did not react like other alkene compounds Structure Before NMR and other spectroscopic tools it was hard to determine the structure of organic compounds Ultimately the symmetry of the molecule revealed its structure All carbon atoms, and all carbon-carbon bonds, are symmetrically equivalent To account for these observations the proposed structure consisted of a cyclic compound stabilized by resonance Each resonance structure is equal in energy and thus each contributes equally to the overall structure Stability The resonance structures imply an extra stability, but the amount of stability in benzene is much more than a typical resonance structure Consider reactivity: RCO3H RCO3H RCO3H O O No reaction The same reactivity behavior is observed for almost any alkene reaction Aromatic Compounds Have More Stability than Conjugated Alkenes Can measure stability by hydrogenation H2 catalyst The energy required for this hydrogenation indicates the stability of the alkene 2 Kcal/mol Conjugation stability 55.4 Kcal/mol 57.4 Kcal/mol Almost double in energy 49.8 Kcal/mol 28.6 Kcal/mol How much energy should be in the hydrogenation of Benzene? Have three double bonds in conjugation, so therefore should expect ~79 Kcal/mol (~24 Kcal/mol more than 55 Kcal/mol for 1,3-cyclohexadiene) Benzene is ~ 30 Kcal/mol more stable than predicted!! Aromatic Stabilization This ~30 Kcal/mol stabilization is called “aromatic stabilization” It is the cause of the difference in reactivity between normal alkenes It would cost ~30 Kcal/mol to break the aromaticity and thus the normal alkene reactions do not occur with benzene Somehow having these three double bonds in resonance in a cyclic system offers a tremendous amount of energy Cyclic system alone, however, is not sufficient for aromatic stabilization Consider a four membered ring Cyclobutadiene also has a ring structure with conjugated double bonds This compound however is highly reactive and does not exist with equivalent single and double bonds In solution it reacts with itself in a Diels-Alder reaction Why the Difference in Stability? Can already see in electron density maps that cyclobutadiene is not symmetric Benzene 6-fold symmetry Cyclobutadiene Not symmetric Consider the Molecular Orbitals for Benzene For benzene there are 6 atomic p orbitals in conjugation therefore there will be 6 MO’s As the number of nodes increase, the energy increases For lowest energy MO there are zero nodes, therefore bonding interactions between each carbon-carbon bond Benzene model Top view with orbitals Side view Entire MO Picture for Benzene 6 nodes 4 nodes 4 nodes E 2 nodes 2 nodes Zero nodes Notice all electrons are in bonding MO’s All the antibonding MO’s are unfilled With a cyclic system we obtain degenerate orbitals (orbitals of the same energy) Overall this electronic configuration is much more stable than the open chain analog This is now the definition of an aromatic compound (not aroma), Flat conjugated cyclic system is MORE stable than the open chain analog Consider Cyclobutadiene E Unlike benzene, cyclobutadiene has two electrons at the nonbonding energy level (these electrons do not stabilize the electronic structure) Antiaromatic Cyclobutadiene is less stable than butadiene < If a cyclic conjugated system is less stable than the open chain analog it is called antiaromatic Part of the reason for cycobutadiene to be antiaromatic is the presence of two MO’s at the nonbonding level In butadiene all electrons are in bonding MO’s therefore the electrons are more stable in butadiene relative to cyclobutadiene Hückel’s Rule In order to determine if a system is aromatic or antiaromatic, without needing to determine the overall electronic energy of the closed form versus the open form, Hückel’s rule was developed First the cyclic system must have a p orbital on all atoms in a continuous cyclic chain (if there is an atom without a p orbital in the cycle then the system is nonaromatic) In practice this means the cyclic system must be flat (to allow overlap of p orbitals) If these criteria are met then: If the system has 4n+2 π electrons, it is aromatic If the system has 4n π electrons, it is antiaromatic Examples 6 π electrons, 4n+2 where n=1 Therefore aromatic 4 π electrons, 4n where n=1 Therefore antiaromatic If there is an atom without a p orbital in the ring, the compound is nonaromatic nonaromatic Remember that the cyclic ring must have overlap of p orbitals to be considered aromatic or antiaromatic Molecule adopts a non-flat low energy conformation top view side view Aromatic Ions Benzene is a neutral aromatic compound Any compound with 4n+2 electrons in a continuous loop is considered aromatic regardless of the number of carbons in the loop There are many aromatic compounds with a different number of electrons than atoms in the loop Due to this difference usually these compounds are ions, hence aromatic ions Cyclopentadienyl Anion Cyclopentadiene is nonaromatic since there is not a p orbital on one of the carbons in the ring base nonaromatic pKa ~16 Upon removal of a proton, however, there is now a p orbital on each carbon 6 electrons in system, therefore according to Hückel this is aromatic Due to this aromaticity cyclopentadienyl anion has unique properties First, the compound is very acidic, pKa of ~16 compared to other allyl positions of ~45 Due to stability of anion once formed Since it is aromatic it is more stable than the open chain anion analog (pentadienyl anion) It will still react with electrophiles in an SN2 reaction According to Hückel’s rule, however, the carbocation should be antiaromatic Cyclopentadienyl cation has only 4 electrons in the continuous loop of p orbitals, therefore it is an antiaromatic compound Since it is antiaromatic it will not form, too high in energy Other Common Aromatic Ions Any compound that will have 4n+2 electrons in a continuous loop for planar conjugated compound will be favored due to aromatic nature Nomenclature of Benzene Derivatives The IUPAC name of 1,3,5-cyclohexatriene is never used The common name of benzene dominates naming of these structures In addition, another common naming tool for benzene derivates is for disubstituted compounds (ortho, meta, para) ortho- dimethylbenzene meta- dimethylbenzene para- dimethylbenzene Other naming follows rules already learned Number along ring to give lowest number First priority substituent is at the 1-position Other common names O CH3 toluene OH phenol OH benzoic acid If the benzene group is being considered as a substituent instead of as a root name, then it is given a phenyl prefix (reason for the word phenol) Another common name is used for the substituted toluene (called benzyl) Heterocyclic Aromatic Compounds Compounds that contain atoms besides carbon can also be aromatic Need to have a continuous loop of orbital overlap and follow Hückel’s rule for the number of electrons in conjugation Common noncarbon atoms to see in aromatic compounds include oxygen, nitrogen, and sulfur Pyridine One common aromatic compound with nitrogen is pyridine N One carbon atom of benzene has been replaced with nitrogen Consider the placement of electrons N Lone pair is orthogonal to conjugated electrons in ring The number of electrons in conjugation is 6 (don’t include lone pair that is orthogonal to ring) therefore pyridine follows Hückel’s rule and is aromatic Pyridine can be protonated in acidic conditions and it will still be aromatic, protonation occurs at lone pair Pyrrole A similar aromatic compound is pyrrole H N N H With pyrrole the lone pair is included in the conjugated ring Have 6 electrons in loop and therefore this compound is aromatic If protonated, however, pyrrole will become nonaromatic since the nitrogen would thus be sp3 hybridized without a p orbital for conjugation Difference in electron placement affects properties pyridine pyrrole Excess electron density of lone pair is localized orthogonal to ring in pyridine while the electron density is conjugated in ring with pyrrole Some other common heterocyclic aromatic compounds All of these compounds have 6 electrons conjugated in ring Consider where the lone pair(s) are located for each heteroatom O S furan thiophene N N pyrimidine N NH imidazole Fused Rings Compounds with more than one fused ring can also be aromatic The simplest two ring fused system is called naphthalene Like benzene, naphthalene is an aromatic compound with 10 electrons in a continuous ring around the cyclic system (one p orbital on each carbon is conjugated) The reactivity of naphthalene is similar to benzene It is unreactive toward normal alkene reactions because any addition would lower the aromatic stabilization If it did react, however, there would still be one benzene ring intact HBr Br Hypothetical reaction – does not occur With larger fused ring systems normal alkene reactions start to occur Anthracene Br Br2 Br Two intact benzene rings Reactions occur at central ring due to large aromatic stabilization remaining NO2 NO2 Diels-Alder reactions can also occur about this central ring Fused Heterocyclics Fused ring systems with heterocyclics can also be aromatic Extremely important compounds biologically and medicinally Two of the four constituents of base pairs in DNA consist of fused aromatic rings, the other two bases, cytosine (C) and thymine (T), are one ring aromatic base pairs Spectroscopy of Aromatic Compounds We have already seen how aromatic benzene compounds have a relatively large downfield NMR shift due to aromatic ring current Therefore any of these aromatic systems, which by definition have a ring current, have a large downfield shift Can use as a characteristic of aromaticity Mass Spectrometry A characteristic peak in a MS for a benzenoid compound is the presence of a peak at m/z 91 (if formation is possible) Due to resonance stabilized benzyl cation