* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download FUNCTIONAL GROUPS
Survey
Document related concepts
Transcript
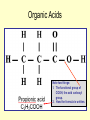
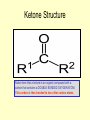
FUNCTIONAL GROUPS FUNCTIONAL GROUPS ARE OUR FRIENDS. Which one is your favorite? Functional Groups Atomic configurations called functional groups, which have decisive influence on the chemical and physical characteristics of the compound; thus those containing the same atomic formations have similar characteristics, which may be: miscibility with water, acidity/alkalinity, chemical reactivity, and oxidation resistance. Organic Acids-Carboxyl Groups • Organic acids are characterized by the functional group R-COOH, where R literally means, “the rest of the compound”. The carbon involved has one double bonded oxygen and a single bonded hydrogen which is also bonded to a hydrogen. Carboxyl Group Because the carbon compound is polar covalent, electrons are not shared equally. This means that the Hydrogen atom is able to be pulled away from the compound, creating a proton, or acid, as shown here. Organic Acids Note two things: 1. The functional group of COOH, the acid carboxyl group. 2. How the formula is written. Amino Acids-note carboxyl group Carboxyl Groups Simple Alcohols Alcohols have the functional group –OH and are usually designated R-OH. One might think that they are a base, however, you have to remember that they are covalently bonded, so they do not form ions. Bases form ions. Note the –OH group attached to the carbon. This substance would be methyl alcohol since it contains one carbon. The formula would be written as CH3OH. Properties of Alcohols The simplest and most commonly used alcohols are methanol and ethanol. Methanol was formerly obtained by the distillation of wood and called "wood alcohol." It is now a cheap commodity, the chemical product of carbon monoxide reacting with hydrogen under high pressure. Methanol is intoxicating but not directly poisonous. It is toxic by its breakdown (toxication) by the enzyme alcohol dehydrogenase in the liver by forming formic acid and formaldehyde which cause permanent blindness by destruction of the optic nerve Alcohols Apart from its familiar role in alcoholic beverages, ethanol is also used as a highly controlled industrial solvent and raw material. To avoid the high taxes on ethanol for consumption, additives are added to make it unpalatable (such as denatonium benzoate — "Bitrex") or poisonous (such as methanol). Ethanol in this form is known generally as denatured alcohol. Alcohols (OH helps it dissolve) Methanol, ethanol, and propanol are miscible in water because the hydroxyl group wins out over the short carbon chain. Butanol, with a four-carbon chain, is moderately soluble because of a balance between the two trends. Alcohols of five or more carbons (Pentanol and higher) are effectively insoluble in water because of the hydrocarbon chain's dominance. Aldehydes • An aldehyde is an organic compound containing a terminal carbonyl group. This functional group, which consists of a carbon atom bonded to a hydrogen atom and double-bonded to an oxygen atom (chemical formula O=CH-), is called the aldehyde group. • The word aldehyde seems to have arisen from alcohol dehydrogenated. Aldehyde Group Missing Oxygen Notice the difference between the aledhyde group and the carboxyl group. Here you should see that the oxygen is missing from the carboxyl group. This gives the compound a totally new set of properites. Uses of Aldehydes Because of their high chemical reactivity, aldehydes are important intermediates for the manufacture of resins, plasticizers, solvents, dyes, and pharmaceuticals. Ketone Structure Notice here that a ketone is an organic compound with a carbon that contains a DOUBLE BONDED OXYGEN ATOM. This carbon is then bonded to two other carbon atoms. Uses of Ketones Ketones are often used in perfumes and paints to stabilize the other ingredients so that they don't degrade as quickly over time. Other uses are as solvents and intermediates in chemical industry. Examples of ketones are acetone, acetophenone, and methyl ethyl ketone. Ketones and Your Body • If you starve for more than a few hours, then the body will run out of glucose (sugar) stores and will switch to breaking down fats and produce 'ketones'. Ketones smell like pear drops and are found in your breath and urine, which is how the body tries to get rid of them. A build up of Ketones in the blood will result in the symptoms that may include a breath odor resembling the smell of fruit. • Insulin is important as it acts as a 'key' to allow sugar to move from the blood into the cells, if you have diabetes and do not have enough insulin then the body behaves as if it has run out of glucose and switches to breaking down fats resulting in ketones. • If this is unrecognised, it can lead to 'diabetic ketoacidosis' (DKA) where you will feel very thirsty, start breathing fast and become very dry and vomit profusely. Esters Properties of Esters Esters, often have a pleasant smell and are found in perfumes, essential oils, and pheromones, and give many fruits their scent. Many esters have distinctive odors, which has led to their use as artificial flavorings and fragrances. See next slide. Ethyl cinnamate cinnamon Ethyl lactate butter, cream Ethyl pentanoate apple Methyl acetate Ethyl acetate peppermint nail polish remover, model paint, model airplane glue Ethers Properties/Uses Ethers are used as anesthetics. The first surgical anesthetic use of ether is credited to Dr. Crawford Williamson Long, MD, age 27, of Jefferson, Georgia. On March 30, 1842, he removed one of the two tumors from the neck of Mr. James Venable under ether anesthesia. AROMATIC COMPOUNDS AROMATIC COMPOUNDS • Aromatic compounds derive their names from the fact that many of these compounds in the early days of discovery were grouped because they were oils with fragrant odors, hence the name aromatic. • The current definition of aromatic compounds includes only those with a benzene ring, which is a special six carbon ring compound with three alternating double bonds. This structure imparts unique properties to benzene which are different from other ring compounds. See the benzene structure on the left. • All aromatic hydrocarbons both benzenoid and non-benzenoid are resonance stabilized. They are water insoluble. They are also carcinogenic (cancer forming) if exposed with sufficient concentration and exposure time. Most have a sweet aroma hence the reason for the name "aromatic". PROBLEM • Benzene represents a special problem in that, to account for all the bonds, there must be alternating double carbon bonds: • One representation is that the structure exists as a superposition of so-called resonance structures, rather than either form individually. • This delocalisation of electrons is known as aromaticity, and gives benzene great stability. Benzene C6H6 Notice that in each structure of Benzene the double bonds have shifted over one carbon. Since the bond shifts so rapidly, both structures essentially exist at the same time. To illustrate a moving bond, scientists represent this moving bond as a circle (next slide). BENZENE RING STRUCTURES BENZENE C6H6 CARBOMER FULLERINES • Fullerenes (Fig 4) are an exciting new classification of aromatic organic molecules that consist of pure carbon. The formula for Fullerenes are either C60 or C70. Review of Functional Groups • Try to draw the group. • • • • • • • Alcohol Acids Benzene Esters Ether Aldehydes Ketones