* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download Bacteria - Hagan Bayley
Survey
Document related concepts
Transcript
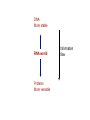
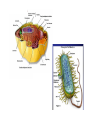
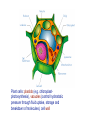
BIOLOGICAL CHEMISTRY Prof. J.H.P. Bayley, Dr. R.M. Adlington and Dr. L. Smith Trinity Term 2007 - First Year Lecture 1 Hagan Bayley Classification, evolution and diversity of life. Overview of classification. Evolution of eukaryotes from bacteria. Development of the nucleus. The evolution of multicellular organisms. Different domains of life: bacteria, archaea, eukaryotes, viruses (different types). [HB] RECOMMENDED TEXTBOOK “Biochemistry” 5th edition 2002 JM Berg, JL Tymoczko, L Stryer Freeman We call this book “Stryer” This week mainly Chapters 1 and 2 MOLECULES OF LIFE WATER solvent PROTEINS NUCLEIC ACIDS CARBOHYDRATES LIPIDS SMALL MOLECULES METAL IONS MACROMOLECULES (POLYMERS) aggregates (bilayers, lipoproteins) WHAT IS LIFE? Self-replicating, autonomous, evolvable, responsive, intelligent • Is a virus alive? Plan, structure, catalysis, energy, generation of variation, communication, computation … provided by … Constituents and major roles: WATER- solvent/ medium PROTEINS- structural and functional, e.g. catalysts NUCLEIC ACIDS- code/ plan CARBOHYDRATES- binding interactions LIPIDS- encapsulation SMALL MOLECULES- energy currency, messengers METAL IONS- energy source, messengers It is difficult to assign specific roles- Nature is opportunistic The importance of chemistry in understanding biology. Covalent bonds hundreds of kJ mole-1 Non-covalent bonds a few to tens of kJ mole-1 Non-covalent bonds Electrostatic Hydrogen bonds (also electrostatic) Van der Waals interactions Although weak, many non-covalent bonds sum to yield significant interactions … e.g. DNA structure Protein folding Binding: enzyme substrates, transmitters and messengers, protein-protein interactions etc. etc. Water Water is a polar molecule- bent structure Water has a cohesive (hydrogen-bonded) structure Weakens non-covalent interactions Competes for hydrogen bonds High dielectric (and salt content) weakens electrostatic interactions ... some important roles of water... • good solvent: high concentrations of polar molecules, including salts, can be obtained • hydrophobic effect - protein folding • barriers- lipid barriers ??? 0 to 100°C- limits to life... but antifreeze proteins, pressure in thermal vents Proteins Built from 20 amino acids Amino acids are chiral: ???why ? L-amino acids, [all are S except cysteine] Peptide bond formation requires energy, kinetically stable (t1/2 = 1000 y) Massive sequence diversity 20n Sequence is specified in genes Proteins fold into 3D structures thus the 1D information of nucleic acids (genes) contains 3D information Structural and functional versatility Protein folding and the “hydrophobic effect” ΔG = ΔH -TΔS ΔG = -RTlnK K = exp(- ΔG/RT) Folding would seem to be entropically disfavored (apparent ordering) • Folded proteins seem to be more ordered • Folded proteins are uniform in structure Water is ordered around hydrophobic molecules When hydrophobic molecules get together: solvent entropy increases Hence hydrophobic amino acid side chains become buried In addition there are numerous non-covalent interactions: electrostatic, hydrogen bonds, van der Waals etc. Overall: finally balanced, folded proteins are barely stabletypically -40 kJ mole-1 for a small 100 amino acid protein Will also meet the hydrophobic effect in lipid bilayer formation … Hydrophobic effect Proteins By virtue of their structural and functional diversity proteins are: Structural components Cytoskeleton Binding molecules receptors antibodies Catalysts enzymes channels, transporters Motors Figure protein Nucleic acids DNA- deoxyribonucleic acid … found in the nuclei of cells … DNA is also a polymer Three components Sugar (deoxyribose) Phosphate 3’5’ phosphodiesters- rather stable to hydrolysis nucleobase Sugar-phosphate backbone Four different bases GATC The four nucleic acid bases (DNA, GATC; RNA,GAUC) Some nomenclature… Nucleobase (see above) e.g. C, cytosine Nucleoside- base connected to sugar e.g. deoxycytidine Nucleotide- phosphorylated derivatives of nucleoside e.g. 5’dCMP adenosine ATP Deoxyguanosine 3’-monophosphate Nucleic acids By virtue of their information content: Encode protein sequences (and structure) Genes (DNA, some RNA viruses) mRNA They also: Act as adapters- tRNA Act as catalysts- ribosomal RNA Regulate gene expression- mi/siRNAs (not in Stryer, but a hot topic) What is RNA?- ribonucleic acid RNA Ribose v deoxyribose DNA is more stable than RNA towards base-catalyzed hydrolysis ???Why? Perhaps this is why it now forms the genome, except in certain viruses (e.g. influenza) Conversion is at the level of the enzyme ribonucleotide reductase NDP dNDP DNA More stable RNA world Proteins More versatile Information flow Carbohydrates (sugars) (CH2O)n Monosaccharides Carbohydrates (sugars) Pentoses and hexoses form rings e.g. glucose Oligosaccharides- again polymers Carbohydrates (sugars) Can be attached to lipids and proteins Tremendous structural diversity is possible Carbohydrates Energy stores, metabolic intermediates In nucleic acids (ribose and deoxyribose) Cell walls in bacteria and plants Attachment to lipid and proteins (recognition by “lectins”) Lipids Example (will return to lipids and membranes later): Phosphatidylcholine Lipids form bilayers, which encapsulate the cells and organelles we will be looking at soon Small molecules small molecules in the cell are often charged with phosphate or carboxylate groups- therefore, they cannot pass through the lipid bilayer energy generation and storage- ATP building blocksUDP-glucose messengers Intracellular- cAMP extracellular e.g. neurotransmittersacetylcholine cofactors- vitamin C ascorbate etc Metal ions Ca2+ as messenger Metalloenzymes, e.g. Ca, Fe, Cu, Zn Ion gradients across membranes, e.g. Na+, K+ Exogenous molecules Poisons, e.g. arsenic Drugs, e.g. Pt carboplatin The elemental composition of cells Four elements constitute approximately 99% of the total number of atoms present in the human body These are hydrogen oxygen carbon nitrogen 62.8 % (of atoms) 25.4 % 9.4 % 1.4 % This largely reflects the high content of water in cells Another seven elements represent approximately 0.9 % of the total number of atoms in the human body: Na, K, Ca, Mg, P, S and Cl Additional metal and non-metals are required by some biological systems, but not by others: The elemental composition of cells is said to be related to the composition of seawater: ??? What do you think? Classification, evolution and diversity of life Overview of classification. Evolution of eukaryotes from bacteria. Development of the nucleus. The evolution of multicellular organisms. Different domains of life: bacteria, archaea, eukaryotes, viruses (different types). In fact, from the biochemical viewpoint, it is striking how similar are all life forms. Classification, evolution and diversity of life Initially, life was classified as either ANIMAL or PLANT However, as new forms of life were discovered, new categories – KINGDOMS – were defined. There were 5 in total – ANIMALIA, PLANTAE, FUNGI, PROTISTA- Eukaryotes BACTERIA- Prokaryotes With new insight into molecular biology (rRNA sequences especially), three DOMAINS of life have been proposed (Carl Woese, 1976): EUKARYA (eukaryotes) Protists, Fungi, Animalia, and Plantae BACTERIA (eubacteria, true bacteria) ARCHAEA 4.5 billion years: recognizable ancestors of bacteria 3.5 billion years ago Eukaryote: a cell or organism composed of cells that have a membrane-bound nucleus and organelles (e.g. mitochondria, chloroplasts) and genetic material organized in chromosomes in which the DNA is combined with histone proteins. Prokaryotic cells contain no nucleus. Prokaryotic cells contain no true membrane bound organelles (e.g. mitochondria). The Bacteria possess the following characteristics: 0. B a cteria are prokaryotic cells . 0. Like the Eukarya, they have membranes composed of unbranched fatty acid chains attached to glycerol by ester linkages 0. The cell walls of Bacteria, unlike the Archaea and the Eukarya, contain peptidoglycan . 0. B a cteria are sensitive to traditional antibacterial antibiotics but are resistant to most antibiotics that affect Eukarya. 0. B a cteria contain rRNA that is unique to the Bacteria as indicated by the presence molecular regions distinctly different from the rRNA of Archaea and Eukarya. 0. Bacteria include mycoplasmas, cyanobacteria, Gram-positive bacteria, and Gram-negative bacteria. The Archaea possess the following characteristics: 0. Archaea are prokaryotic cells . 0. Unlike the Bacteria and the Eukarya, the Archaea have membranes composed of branched hydrocarbon chains attached to glycerol by ether linkages. 0. The cell walls of Archaea contain no peptidoglycan . 0. Archaea are not sensitive to some antibiotics that affect the Bacteria, but are sensitive to some antibiotics that affect the Eukarya. 0. Archaea contain rRNA that is unique to the Archaea as indicated by the presence molecular regions distinctly different from the rRNA of Bacteria and Eukarya. 0. Archaea often live in extreme environments and include methanogens, extreme halophiles, and hyperthermophiles (record 113°C), acidophiles (some at pH 1!) The Eukarya (also spelled Eucarya) possess the following characteristics: 0. E u karya have eukaryotic cells . 0. Like the Bacteria, they have membranes composed of unbranched fatty acid chains attached to glycerol by ester linkages. 0. Not all Eukarya possess cells with a cell wall, but for those Eukarya having a cell wall, that wall contains no peptidoglycan . 0. E u karya are resistant to traditional antibacterial antibiotics but are sensitive to antibiotics that affect eukaryotic cells. 0. E u karya contain rRNA that is unique to the Eukarya as indicated by the presence molecular regions distinctly different from the rRNA of Archaea and Bacteria. The Eukarya are subdivided into the following kingdoms: a. Protista Kingdom Protista are simple, predominately unicellular eukaryotic organisms. Examples includes slime molds, euglenoids, algae, and protozoans. b. Fungi Kingdom Fungi are unicellular or multicellular organisms with eukaryotic cell types. The cells have cell walls but are not organized into tissues. They do not carry out photosynthesis and obtain nutrients through absorption. Examples include sac fungi (e.g. Morel), club fungi (e.g. common mushrooms), yeasts, and molds. c. Plantae Kingdom Plants are multicellular organisms composed of eukaryotic cells. The cells are organized into tissues and have cell walls. They obtain nutrients by photosynthesis and absorption. Examples include mosses, ferns, conifers, and flowering plants. d. Animalia Kingdom Animals are multicellular organisms composed of eukaryotic cells. The cells are organized into tissues and lack cell walls. They do not carry out photosynthesis and obtain nutrients primarily by ingestion. Examples include sponges, worms, insects, and vertebrates. The major organelles of a eukaryotic cell are: NUCLEUS – contains the chromosomes, which consist of DNA and histones. Gene replication. mRNA synthesis. Ribosome production. MITOCHONDRIA – principal function is the production of ATP ENDOPLASMIC RETICULUM: ROUGH – studded with ribosomes- sites of protein synthesis for membrane and secreted proteins SMOOTH –steroid hormone biosynthesis, Ca2+ storage LYSOSOMES - contain hydrolytic enzymes PEROXISOMES - contain oxidative enzymes The lysosomes and peroxisomes degrade foreign substances that have been brought into the cell (simplification) GOLGI COMPLEXES –newly biosynthesised proteins are processed here (post-translational modification), e.g. glycosylated Plant cells: plastids (e.g. chloroplastphotosynthesis), vacuoles (control hydrostatic pressure through fluid uptake, storage and breakdown of molecules), cell wall Origin of organelles Membrane invaginations followed by specialization: nucleus, endoplasmic reticulum, Golgi etc. Endosymbiosis: mitochondria, chloroplasts- ~1 billion years ago Evidence: inner membrane contains similar enzymes and transport systems; contain their own DNA (no histones), related to that found in bacteria; ribosomes have similar biochemical characteristics to those in bacteria, rRNA sequence similarities VIRUSES Viruses are packets of infectious nucleic acids (DNA or RNA) surrounded by protective protein coats. They are unable to generate metabolic energy or synthesise proteins Diseases caused by viruses include the common cold, measles, smallpox, polio and AIDS Viruses have genes and show inheritance, but are reliant on host cells to produce new generations of viruses. Because viruses are dependent on host cells for their replication they are generally not classified as "living". Whether they are "alive", they are obligate parasites, and have no form which can reproduce independently of their host. Like most parasites, they have a specific host range, sometimes specific to one species (or even limited cell types of one species) and sometimes more general. PRIONS A prion is an infectious agent made only of protein. Prions are abnormally-structured forms (amyloid) of a host protein, which are able to convert normal molecules of the protein into the abnormal structure. Prions cause diseases such as BSE (mad cow disease). Lambda bacteriophage: The viral DNA molecule is contained within an icosahedral protein coat to form the head The DNA is injected into the bacterial host through the tail The tips of the tail fibers bind to specific sites on the outer membrane of the host Influenza Negative strand RNA virus Eight separate genes 8 min/ frame Total time 12 h 1.4 mm field Multicellular organisms Differentiation and specialization differentiated cells are genetically identical but have different properties because of differential gene expression Cell communication Primitive example: slime mold Dictyostelium (not really a mold) Exist as single cells in a nutrient rich environment Form aggregrates when starved (signal is extracellular cAMP); cAMP binds to extracellular receptors, triggers release of more cAMP and movement to higher cAMP concentrations, promotes aggregration Formation of fruiting body that can be carried by the wind. A human contains about 1014 cells begins as a single cell regulated gene expression cell division cell migration programmed cell death (apoptosis) responses to neighboring and distant cells Caenorhabditis elegans- nematode worm completely programmed 959 cells The biochemistry underlying all organisms has a common origin and was largely fixed early in evolution. Important processes usual exist and can be studied in lower organisms.