* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download The Heart Lecture Outline
Survey
Document related concepts
Transcript
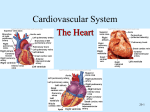
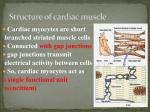
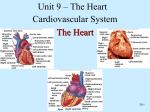
CHAPTER 18 The Cardiovascular System: The Heart Lecture Outline I. The Pulmonary and Systemic Circuits (p. 659; Fig. 18.1) A. The right side of the heart receives oxygen-poor blood returning from body tissues and pumps it to the lungs to release CO2 and pick up O2 (p. 659; Fig. 18.1). 1. The left side of the heart receives oxygenated blood from the lungs and pumps it out to the body to supply oxygen to tissues. 2. The heart has two receiving chambers, the right and left atria, and two pumping chambers, the right and left ventricles. II. Heart Anatomy (pp. 659–671; Figs. 18.2–18.11) A. Size, Location, and Orientation (p. 659; Fig. 18.2) 1. The heart is the size of a fist and weighs 250–300 grams. 2. The heart is found in the mediastinum and two-thirds lies left of the midsternal line. 3. The base is directed toward the right shoulder and the apex points toward the left hip. B. Coverings of the Heart (pp. 660–661; Fig. 18.3) 1. The heart is enclosed in a double-walled sac called the pericardium. a. The superficial pericardium is the fibrous pericardium that protects the heart, anchors it to surrounding structures, and prevents the heart from overfilling. b. Deep to the fibrous pericardium is the serous pericardium, consisting of a parietal layer that lines the inside of the pericardium, and a visceral layer (epicardium) that covers the surface of the heart. 2. Between the visceral and parietal layers is the pericardial cavity, containing a film of serous fluid that lubricates their movement against each other. C. Layers of the Heart Wall (pp. 661–662; Figs. 18.3–18.4) 1. The epicardium is the visceral pericardium of the serous pericardium. 2. The myocardium is composed mainly of cardiac muscle and forms the bulk of the heart. a. Within the myocardium exists a network of connective tissue fibers, the cardiac skeleton, which reinforces the myocardium, supports the heart valves and provides electrical insulation between areas of the heart. 3. The endocardium lines the chambers of the heart and is continuous with the endothelial linings of the vascular system. D. Chambers and Associated Great Vessels (p. 662; Fig. 18.5) 1. There are partitions that separate the heart longitudinally: the interatrial septum divides the atria, and between the ventricles lies the interventricular septum. 2. The right and left atria are the receiving chambers of the heart and only minimally contract to propel blood into the ventricles. a. There are three veins that enter the right atrium: the superior and inferior vena cavae, which return blood from the body, and the coronary sinus, which returns blood from the myocardium. b. Four pulmonary veins enter the left atrium from the lungs. 3. The right ventricle pumps blood into the pulmonary trunk; the left ventricle pumps blood into the aorta. E. Heart Valves (pp. 662–668; Figs. 18.5–18.8) 1. There are two atrioventricular (AV) valves between each atrial-ventricular junction: the tricuspid valve in the right and the mitral valve in the left. a. When the heart is relaxed, the AV valves hang loosely down into the ventricles. b. When the ventricles contract, blood is forced upward against the flaps of the AV valves, pushing them closed. c. Each flap of the AV valves are anchored to papillary muscles in the ventricles by collagen strings called chordae tendinae, which prevent eversion of the valves into the atria. 2. Aortic and pulmonary semilunar (SL) valves are located at the base of the arteries exiting the heart and prevent backflow of blood into the ventricles. a. When ventricular pressure rises above aortic and pulmonary pressure, the SL valves are forced open, allowing blood to be ejected from the heart. b. When the ventricles relax, blood flows backward toward the heart, filling the cusps of the SL valves, forcing them closed. 3. There are no valves at the entrances of the vena cavae or pulmonary veins, because the intertia of blood and the collapse of the atria during contraction minimizes backflow into these vessels. F. Pathway of Blood Through the Heart (p. 668; Figs. 18.9–18.10) 1. The right side of the heart pumps blood into the pulmonary circuit; the left side of the heart pumps blood into the systemic circuit. 2. Equal volumes of blood are pumped to the pulmonary and systemic circuits at the same time, but the two sides have different workloads. a. The right ventricle is thinner walled than the left and is a short, low-pressure circulation. b. The left ventricle is three times thicker than the right, enabling it to generate a much higher pressure, so that the force can overcome the much greater resistance in the systemic circulation. G. Coronary Circulation (pp. 668–671; Fig. 18.11) 1. The heart receives no nourishment from the blood as it passes through the chamber, so a series of vessels, the coronary circulation, exist to supply blood to the heart itself. a. The left coronary artery gives rise to the anterior interventricular artery and the circumflex artery, which supply blood to the left ventricle and atrium. b. The right coronary artery branches into the right marginal artery and the posterior interventricular artery, which supply blood to the right ventricle and atrium. c. Cardiac veins collect blood from the capillary beds of the myocardium and drain into the coronary sinus, which empties into the right atrium. d. The great cardiac vein, middle cardiac vein, and small cardiac vein all empty into the coronary sinus. 2. In a myocardial infarction, there is prolonged coronary blockage that leads to cell death. III. Cardiac Muscle Fibers (pp. 671–674; Figs. 18.12–18.13) A. Microscopic Anatomy (p. 671; Fig. 18.12) 1. Cardiac muscle is striated and contraction occurs via the sliding filament mechanism. 2. The cells are short, fat, and branched, and each cardiac muscle fiber has one or two large, pale, centrally located nuclei. 3. Intercellular space is filled with a matrix of loose connective tissue that connects the muscle to the cardiac skeleton. 4. Cells are connected to each other at intercalated discs, containing desmosomes for structural strength, and gap junctions that allow electrical current to travel from cell to cell. 5. Cardiac muscle cells have large mitochondria that occupy 25–35% of the total cell volume, have myofibrils arranged in sarcomeres, but have a less extensive sarcoplasmic reticulum, when compared to skeletal muscle. B. Mechanism and Events of Contraction (pp. 671–673; Fig. 18.13) 1. Some cardiac muscle cells are self-excitable and initiate their own depolarization, as well as depolarizing the rest of the heart. 2. The heart contracts as a unit or not at all because, unlike skeletal muscle motor units, gap junctions electrically tie all cardiac muscle cells together. 3. The heart’s absolute refractory period is longer than a skeletal muscle’s, preventing tetanic contractions. 4. Although the basic contraction mechanism is the same between cardiac and skeletal muscle cells, the events that trigger contraction differ, with roughly 20% of the Ca++ involved entering the cell from the extracellular space. 5. The mechanism of stimulation of cardiac muscle contraction is as follows: a. When cardiac muscle cells are stimulated, voltage gated Na+ channels allow Na+ to enter the cell, resulting in rapid depolarization. b. Depolarization opens slow Ca++ channels, allowing Ca++ to enter the cell, even as K+ exits, producing a plateau phase of the action potential that delays repolarization. c. After 200 msec, Ca++ channels are inactivated, K+ channels open, and the cell rapidly repolarizes back to the resting membrane potential. C. Energy Requirements (pp. 673–674) 1. Cardiac muscle has more mitochondria than skeletal muscle, indicating reliance on exclusively aerobic respiration for its energy demands. 2. Cardiac muscle is capable of switching nutrient pathways to use whatever nutrient supply is available, including lactic acid. IV. Heart Physiology (pp. 674–685; Figs. 18.14–18.23) A. Electrical Events (pp. 674–678; Figs. 18.14–18.19) 1. The heart does not depend on the nervous system to provide stimulation; it relies on gap junctions to conduct impulses throughout the heart and the intrinsic conduction system, which are specialized cardiac cells that initiate and distribute impulses to ensure that the heart depolarizes in an orderly fashion. 2. The cardiac pacemaker cells have an unstable resting potential and produce pacemaker potentials that continuously depolarize, initiating the action potentials that are conducted throughout the heart. 3. Impulses pass through the cardiac pacemaker cells in the following order: sinoatrial node, atrioventricular node, atrioventricular bundle, right and left bundle branches, and the subendocardial conducting network. a. The sinoatrial node is located in the right atrium and is the primary pacemaker for the heart. b. The atrioventricular (AV) node, in the interatrial septum, delays firing slightly, in order to allow the atria to finish contracting before the ventricles contract. c. The atrioventricular (AV) bundle is the only electrical connection between the atria and the ventricles and conducts impulses into the ventricles from the AV node. d. The right and left bundle branches conduct impulses down the interventricular septum to the apex. e. The subendocardial conducting network penetrates throughout the ventricular walls, distributing impulses throughout the ventricles. 4. The autonomic nervous system modifies the heartbeat through cardiac centers in the medulla oblongata: a. The cardioacceleratory center projects to sympathetic neurons throughout the heart, increasing both heart rate and contractile force. b. The cardioinhibitory center sends impulses to the parasympathetic dorsal vagus nucleus in the medulla oblongata, which stimulates the vagus nerve to the heart, slowing the heartbeat. 5. An electrocardiograph monitors and amplifies the electrical signals of the heart and records it as an electrocardiogram (ECG). a. A typical ECG has three deflections: a P wave, indicating depolarization of the atria; a QRS complex, resulting from ventricular contraction; and a T wave, caused by ventricular repolarization. B. Heart Sounds (pp. 678–679; Fig. 18.20) 1. Normal a. The first heart sound, lub, corresponds to closure of the AV valves, and occurs during ventricular systole. b. The second heart sound, dup, corresponds to the closure of the aortic and pulmonary valves and occurs during ventricular diastole. 2. Abnormal a. Heart murmurs are extraneous heart sounds due to turbulent backflow of blood through a valve that does not close tightly. C. Mechanical Events: The Cardiac Cycle (pp. 679–681; Fig. 18.21) 1. Systole is the contractile phase of the cardiac cycle and diastole is the relaxation phase of the cardiac cycle. 2. A cardiac cycle consists of a series of pressure and volume changes in the heart during one heartbeat. a. Ventricular filling occurs during mid-to-late ventricular diastole, when the AV valves are open, semilunar valves are closed, and blood is flowing passively into the ventricles. b. The atria contract during the end of ventricular diastole, propelling the final volume of blood into the ventricles. c. The atria relax and the ventricles contract during ventricular systole, causing closure of the AV valves and opening of the semilunar valves, as blood is ejected from the ventricles to the great arteries. d. Isovolumetric relaxation occurs during early diastole, resulting in a rapid drop in ventricular pressure, which then causes closure of the semilunar valves and opening of the AV valves. D. Cardiac Output (pp. 681–685; Figs. 18.22–18.23) 1. Cardiac output is defined as the amount of blood pumped out of a ventricle per beat and is calculated as the product of stroke volume and heart rate. a. Stroke volume is the volume of blood pumped out of a ventricle per beat, and roughly equals 70 ml. b. The average adult heart rate is 75 beats per minute. c. Cardiac output changes with demand; cardiac reserve is the difference between the resting and maximal cardiac output. 2. Regulation of Stroke Volume a. Stroke volume represents the difference between the end diastolic volume (EDV), the amount of blood that collects in the ventricle during diastole, and end systolic volume (ESV), the volume of blood that remains in the ventricle after contraction is complete. b. The Frank-Starling law of the heart states that the critical factor controlling stroke volume is preload, the degree of stretch of cardiac muscle cells immediately before they contract. c. The most important factor determining the degree of stretch of cardiac muscle is venous return to the heart. d. Contractility is the contractile strength achieved at a given muscle length; contractile strength increases if there is an increase in cytoplasmic calcium ion concentration. e. Afterload is the ventricular pressure that must be overcome before blood can be ejected from the heart and does not become a significant determinant of stroke volume except in hypertensive individuals. 3. Regulation of Heart Rate a. Sympathetic stimulation of pacemaker cells increases heart rate and contractility by increasing Ca++ movement into the cell. b. Parasympathetic inhibition of cardiac pacemaker cells decreases heart rate by increasing membrane permeability to K+. c. Hormones such as epinephrine and thyroxine increase heart rate. d. Ion imbalances can interfere with the normal function of the heart. e. Age, gender, exercise, and body temperature all influence heart rate. 4. Homeostatic Imbalance of Cardiac Output a. Congestive heart failure occurs when the pumping efficiency of the heart is so low that blood circulation cannot meet tissue needs. b. Pulmonary congestion occurs when one side of the heart fails, resulting in pulmonary edema. V. Developmental Aspects of the Heart (pp. 685–687; Figs. 18.24–18.25) A. Before Birth (pp. 685–686; Figs. 18.24–18.25) 1. The heart begins as a pair of endothelial tubes that fuse to make a single heart tube with four bulges representing the four chambers. 2. The foramen ovale is an opening in the interatrial septum that allows blood returning to the pulmonary circuit to be directed into the atrium of the systemic circuit. 3. The ductus arteriosus is a vessel extending between the pulmonary trunk and the aortic arch that allows blood in the pulmonary trunk to be shunted to the aorta. B. Heart Function Throughout Life (pp. 686–687) 1. Regular exercise causes the heart to enlarge and become more powerful and efficient. 2. Sclerosis and thickening of the valve flaps occurs over time, in response to constant pressure of the blood against the valve flaps. 3. Decline in cardiac reserve occurs due to a decline in efficiency of sympathetic stimulation. 4. Fibrosis of cardiac muscle may occur in the nodes of the intrinsic conduction system, resulting in arrhythmias. 5. Atherosclerosis is the gradual deposit of fatty plaques in the walls of the systemic vessels. CHAPTER 19 The Cardiovascular System: Blood Vessels Lecture Outline Part 1: Blood Vessel Structure and Function (pp. 693–701; Figs. 19.1– 19.5; Table 19.1) I. Structure of Blood Vessel Walls (p. 693; Figs. 19.1–19.2; Table 19.1) A. The walls of all blood vessels except the smallest consist of three layers: the tunica intima, tunica media, and tunica externa (p. 693; Fig. 19.1). B. The tunica intima reduces friction between the vessel walls and blood; the tunica media controls vasoconstriction and vasodilation of the vessel; and the tunica externa protects, reinforces, and anchors the vessel to surrounding structures (p. 693; Fig. 19.2; Table 19.1). II. Arterial System (pp. 693–696; Fig. 19.2; Tables 19.1–19.2) A. Elastic, or conducting, arteries contain large amounts of elastin, which enables these vessels to withstand and smooth out pressure fluctuations due to heart action (pp. 693– 695; Fig. 19.2; Table 19.1). B. Muscular, or distributing, arteries deliver blood to specific body organs and have the greatest proportion of tunica media of all vessels, making them more active in vasoconstriction (p. 696; Table 19.1). C. Arterioles are the smallest arteries and regulate blood flow into capillary beds through vasoconstriction and vasodilation (p. 696). III. Capillaries (pp. 696–698; Figs. 19.3–19.4; Table 19.1) A. Capillaries are the smallest vessels and allow for exchange of substances between the blood and interstitial fluid (pp. 696–698; Fig. 19.3; Table 19.1). 1. Continuous capillaries are most common and allow passage of fluids and small solutes. 2. Fenestrated capillaries are more permeable to fluids and solutes than continuous capillaries. 3. Sinusoid capillaries are leaky capillaries that allow large molecules to pass between the blood and surrounding tissues. B. Capillary beds are microcirculatory networks consisting of a vascular shunt and true capillaries, which function as the exchange vessels (p. 698; Fig. 19.4). C. A cuff of smooth muscle, called a precapillary sphincter, surrounds each capillary at the metarteriole and acts as a valve to regulate blood flow into the capillary (p. 698; Fig. 19.4). IV. Venous System (pp. 698–699; Fig. 19.5; Table 19.1) A. Venules are formed where capillaries converge and allow fluid and white blood cells to move easily between the blood and tissues (p. 698; Table 19.1). B. Venules join to form veins, which are relatively thin-walled vessels with large lumens containing about 65% of the total blood volume (pp. 698–699; Fig. 19.5; Table 19.1). V. Vascular Anastomoses (pp. 699–701) A. Vascular anastomoses form where vascular channels unite, allowing blood to be supplied to and drained from an area even if one channel is blocked (p. 699). Part 2: Physiology of Circulation (pp. 701–720; Figs. 19.6–19.18; Table 19.2) VI. Introduction to Blood Flow, Blood Pressure, and Resistance (pp. 701–702) A. Blood flow is the volume of blood flowing through a vessel, organ, or the entire circulation in a given period and may be expressed as ml/min (p. 701). B. Blood pressure is the force per unit area exerted by the blood against a vessel wall and is expressed in millimeters of mercury (mm Hg) (pp. 701–702). C. Resistance is a measure of the friction between blood and the vessel wall and arises from three sources: blood viscosity, blood vessel length, and blood vessel diameter (p. 702). D. Relationship Between Flow, Pressure, and Resistance (p. 702) 1. If blood pressure increases, blood flow increases; if peripheral resistance increases, blood flow decreases. 2. Peripheral resistance is the most important factor influencing local blood flow, because vasoconstriction or vasodilation can dramatically alter local blood flow, while systemic blood pressure remains unchanged. VII. Systemic Blood Pressure (pp. 702–704; Figs. 19.6–19.7) A. The pumping action of the heart generates blood flow; pressure results when blood flow is opposed by resistance (p. 702). B. Systemic blood pressure is highest in the aorta and declines throughout the pathway until it reaches 0 mm Hg in the right atrium (pp. 702–703; Fig. 19.6). C. Arterial blood pressure reflects how much the arteries close to the heart can be stretched (compliance, or distensibility) and the volume forced into them at a given time (p. 703; Fig. 19.6). 1. When the left ventricle contracts, blood is forced into the aorta, producing a peak in pressure called systolic pressure (120 mm Hg). 2. Diastolic pressure occurs when blood is prevented from flowing back into the ventricles by the closed semilunar valve and the aorta recoils (70–80 mm Hg). 3. The difference between diastolic and systolic pressure is called the pulse pressure. 4. The mean arterial pressure (MAP) represents the pressure that propels blood to the tissues. D. Capillary blood pressure is low, ranging from 15–40 mm Hg, which protects the capillaries from rupture but is still adequate to ensure exchange between blood and tissues (p. 703; Fig. 19.6). E. Venous blood pressure changes very little during the cardiac cycle and is low, reflecting cumulative effects of peripheral resistance (pp. 703–704; Fig. 19.7). VIII. Maintaining Blood Pressure (pp. 704–711; Figs. 19.8–19.12; Table 19.2) A. Blood pressure varies directly with changes in blood volume and cardiac output, which are determined primarily by venous return and neural and hormonal controls (p. 704; Fig. 19.8). B. Short-term neural controls of peripheral resistance alter blood distribution to meet specific tissue demands and maintain adequate MAP by altering blood vessel diameter (pp. 704–707; Fig. 19.9). 1. Clusters of neurons in the medulla oblongata, the cardioacceleratory, cardioinhibitory, and vasomotor centers, form the cardiovascular center that regulates blood pressure by altering cardiac output and blood vessel diameter. 2. Baroreceptors detect stretch and send impulses to the vasomotor center, inhibiting its activity and promoting vasodilation of arterioles and veins. 3. Chemoreceptors detect a rise in carbon dioxide levels of the blood and stimulate the cardioacceleratory and vasomotor centers, which increases cardiac output and vasoconstriction. 4. The cortex and hypothalamus can modify arterial pressure by signaling the medullary centers. C. Chemical controls influence blood pressure by acting on vascular smooth muscle or the vasomotor center (p. 707; Table 19.2). 1. Norepinephrine and epinephrine promote an increase in cardiac output and generalized vasoconstriction. 2. Atrial natriuretic peptide acts as a vasodilator and an antagonist to aldosterone, resulting in a drop in blood volume. 3. Antidiuretic hormone promotes vasoconstriction and water conservation by the kidneys, resulting in an increase in blood volume. 4. Angiotensin II acts as a vasoconstrictor, as well as promoting the release of aldosterone and antidiuretic hormone. 5. Endothelium-derived factors promote vasoconstriction and are released in response to low blood flow. 6. Nitric oxide is produced in response to high blood flow or other signaling molecules and promotes systemic and localized vasodilation. 7. Inflammatory chemicals, such as histamine, prostacyclin, and kinins, are potent vasodilators. 8. Alcohol inhibits antidiuretic hormone release and the vasomotor center, resulting in vasodilation. D. Long-Term Regulation: Renal Mechanisms (pp. 708–710; Figs. 19.10–19.11) 1. The direct renal mechanism counteracts an increase in blood pressure by altering blood volume, which increases the rate of kidney filtration. 2. The indirect renal mechanism is the renin-angiotensin-aldosterone mechanism, which counteracts a decline in arterial blood pressure by causing systemic vasoconstriction. E. Monitoring circulatory efficiency is accomplished by measuring pulse and blood pressure; these values together with respiratory rate and body temperature are called vital signs (p. 710; Fig. 19.12). 1. A pulse is generated by the alternating stretch and recoil of elastic arteries during each cardiac cycle. 2. Systemic blood pressure is measured indirectly using the auscultatory method, which relies on the use of a blood pressure cuff to alternately stop and reopen blood flow into the brachial artery of the arm. F. Alterations in blood pressure may result in hypotension (low blood pressure) or transient or persistent hypertension (high blood pressure) (pp. 710–711). IX. Blood Flow Through Body Tissues: Tissue Perfusion (pp. 711–720; Figs. 19.13–19.18) A. Tissue perfusion is involved in delivery of oxygen and nutrients to, and removal of wastes from, tissue cells; gas exchange in the lungs; absorption of nutrients from the digestive tract; and urine formation in the kidneys (pp. 711–712; Fig. 19.13). B. Velocity or speed of blood flow changes as it passes through the systemic circulation; it is fastest in the aorta and declines in velocity as vessel diameter decreases (p. 712; Fig. 19.14). C. Autoregulation: Local Regulation of Blood Flow (pp. 712–713; Fig. 19.15) 1. Autoregulation is the automatic adjustment of blood flow to each tissue in proportion to its needs and is controlled intrinsically by modifying the diameter of local arterioles. 2. Metabolic controls of autoregulation are most strongly stimulated by a shortage of oxygen at the tissues. 3. Myogenic control involves the localized response of vascular smooth muscle to passive stretch. 4. Long-term autoregulation develops over weeks or months and involves an increase in the size of existing blood vessels and an increase in the number of vessels in a specific area, a process called angiogenesis. D. Blood Flow in Special Areas (pp. 713–716) 1. Blood flow to skeletal muscles varies with level of activity and fiber type. 2. Muscular autoregulation occurs almost entirely in response to decreased oxygen concentrations. 3. Cerebral blood flow is tightly regulated to meet neuronal needs, because neurons cannot tolerate periods of ischemia, and increased blood carbon dioxide causes marked vasodilation. 4. In the skin, local autoregulatory events control oxygen and nutrient delivery to the cells, while neural mechanisms control the body temperature regulation function. 5. Autoregulatory controls of blood flow to the lungs are the opposite of what happens in most tissues: low pulmonary oxygen causes vasoconstriction, while higher oxygen causes vasodilation. 6. Movement of blood through the coronary circulation of the heart is influenced by aortic pressure and the pumping of the ventricles. E. Blood Flow Through Capillaries and Capillary Dynamics (pp. 716–717; Figs. 19.16– 19.17) 1. Vasomotion, the slow, intermittent flow of blood through the capillaries, reflects the action of the precapillary sphincters in response to local autoregulatory controls. 2. Capillary exchange of nutrients, gases, and metabolic wastes occurs between the blood and interstitial space through diffusion. 3. Hydrostatic pressure (HP) is the force of a fluid against a membrane. 4. Colloid osmotic pressure (OP), the force opposing hydrostatic pressure, is created by the presence of large, nondiffusible molecules that are prevented from moving through the capillary membrane. 5. Fluids will leave the capillaries if net HP exceeds net OP, but fluids will enter the capillaries if net OP exceeds net HP. F. Circulatory shock is any condition in which blood volume is inadequate and cannot circulate normally, resulting in blood flow that cannot meet the needs of a tissue (p. 717; Fig. 19.18). 1. Hypovolemic shock results from a large-scale loss of blood, and may be characterized by an elevated heart rate and intense vasoconstriction. 2. Vascular shock is characterized by a normal blood volume accompanied by extreme vasodilation, resulting in poor circulation and a rapid drop in blood pressure. 3. Transient vascular shock is due to prolonged exposure to heat, such as while sunbathing, resulting in vasodilation of cutaneous blood vessels. 4. Cardiogenic shock occurs when the heart is too inefficient to sustain normal blood flow and is usually related to myocardial damage, such as repeated myocardial infarcts. CHAPTER 20 The Lymphatic System and Lymphoid Organs and Tissues Lecture Outline I. Lymphatic System (pp. 752–754; Figs. 20.1–20.2) A. The fluid that does not circulate back into the blood from the interstitial fluid is collected by the lymphatic vessels (p. 752). B. The lymphatic vessels form a one-way system in which lymph flows only toward the heart (p. 752). 1. The lymphatic transport system starts with the highly permeable lymphatic capillaries, found between the tissue cells and blood capillaries, in the loose connective tissue (pp. 752–753; Fig. 20.1). a. Large molecules, such as proteins, that are too large to enter blood capillaries can easily pass into lymphatic capillaries. 2. The lymph capillaries flow into the collecting lymphatic vessels and carry the lymph to the lymphatic trunks. 3. The lymphatic trunks drain fairly large areas of the body and eventually empty the lymph back into the circulatory system via the thoracic duct or the right lymphatic duct (p. 753; Fig. 20.2) C. Lymphatic vessels are low-pressure vessels that use the same mechanisms as veins to return the lymph to the circulatory system—skeletal muscle compression, pressure changes during breathing, and valves to prevent backflow (p. 754). II. Lymphoid Cells and Tissues (pp. 754–755; Fig. 20.3) A. Lymphoid Cells (p. 754; Fig. 20.3) 1. Lymphocytes arise in the red bone marrow and mature into one of two lymphocytes: T cells, or B cells. 2. Macrophages play an important role in body protection by acting as phagocytes and in activating T lymphocytes. 3. Dendritic cells, found in lymphoid tissue, also play a role in T lymphocyte activation. 4. Reticular cells produce the stroma, which is the network that supports the other cell types in the lymphoid tissue. B. Lymphoid tissues house and provide a proliferation site for lymphocytes and furnish an ideal surveillance site for lymphocytes and macrophages (pp. 754–755; Fig. 20.3). 1. Lymphoid tissues may be diffuse lymphoid tissues, found in nearly every body organ, or lymphoid follicles, which may form part of larger lymphoid organs or be found as aggregations, such as the Peyer’s patches in the intestinal wall. III. Lymph Nodes (pp. 755–757; Fig. 20.4) A. The principal lymphoid organs in the body are the lymph nodes, which act as filters to remove and destroy microorganisms and other debris for the lymph before it is transported back to the bloodstream (p. 755). B. Each lymph node is surrounded by a dense fibrous capsule with an internal framework, or stroma, of reticular fibers that supports the lymphocytes (pp. 755–756; Fig. 20.4). C. Lymph enters the convex side of a lymph node through afferent lymphatic vessels and exits via fewer efferent vessels, after passing through several sinuses (pp. 755–756; Fig. 20.4). 1. There are more afferent vessels than efferent vessels, so that the lymph gets backed up slightly in the lymph node, giving the lymphocytes and macrophages time to perform their defensive functions. IV. Other Lymphoid Organs (pp. 757–759; Figs. 20.5–20.9) A. The spleen is the largest lymphoid organ, located in the left side of the abdominal cavity directly below the diaphragm (pp. 757–758; Figs. 20.5–20.6). 1. The spleen’s main function is to remove old and defective RBCs and platelets as well as foreign matter and debris from the blood. It also provides a site for lymphocyte proliferation and immune surveillance. 2. The spleen is surrounded by a fibrous capsule and contains both lymphocytes found in white pulp and macrophages found in red pulp. B. Thymus (pp. 758–759; Figs. 20.5, 20.7) 1. The thymus secretes hormones that cause T lymphocytes to become immunocompetent. 2. The thymus is made up of thymic lobules containing an outer cortex and an inner medulla. 3. The thymus differs from other lymphoid organs in that it has no B cells and, therefore, no follicles, it is not directly involved in fighting antigens, and the stroma of the thymus consists of epithelial cells, not reticular fibers. C. Mucosa-associated lymphoid tissues, MALT, are a set of lymphoid tissues located in mucous membranes throughout the body (p. 759; Figs. 20.8–20.9). 1. Tonsils are the simplest lymphoid organs—forming a ring of lymphoid tissue around the opening to the pharynx—which serve to gather and remove many of the pathogens entering the pharynx in food or inhaled air. 2. Clusters of lymphoid follicles, the Peyer’s patches, are found in the wall of the distal portion of the small intestine. 3. The appendix is located off the first part of the large intestine and contains large numbers of lymphoid follicles. CHAPTER 21 The Immune System: Innate and Adaptive Body Defenses Lecture Outline Part 1: Innate Defenses (pp. 765–773; Figs. 21.1–21.6; Tables 21.1–21.2) I. Surface Barriers: Skin and Mucosae (pp. 765–766; Fig. 21.1) A. Skin, a highly keratinized epithelial membrane, represents a physical barrier to most microorganisms and their enzymes and toxins (p. 765). B. Mucous membranes line all body cavities open to the exterior and function as an additional physical barrier (p. 765). C. Protective chemicals of the epithelial tissues include acid to inhibit bacterial growth, enzymes (lysozyme) to destroy microorganisms, mucin to create sticky traps, defensins as antimicrobial peptides, and other specialized regional secretions (pp. 765–766). II. Internal Innate Defenses: Cells and Chemicals (pp. 766–773; Figs. 21.2–21.6; Tables 21.1–21.2) A. Phagocytes, such as neutrophils and macrophages, confront microorganisms that breach the external barriers (p. 766; Fig. 21.2). 1. Phagocytes must be able to adhere to a pathogen before it can engulf it; to counteract this, pathogens are coated with complement proteins called opsonins. 2. Neutrophils and macrophages destroy pathogens by engulfing them, acidifying the phagolysosome (pathogen-containing vesicles associated with a lysosome within the phagocyte), and digesting the contents with lysosomal enzymes. 3. When phagocytes cannot ingest their targets, they may release chemicals lethal to pathogens. B. Natural killer cells are able to lyse and kill cancer cells and virally infected cells before the adaptive immune system has been activated (pp. 766–767). C. Inflammation occurs any time the body tissues are injured by physical trauma, intense heat, irritating chemicals, or infection by viruses, fungi, or bacteria (pp. 767–771; Figs. 21.3–21.4; Table 21.1). 1. Inflammation is beneficial because it prevents the spread of damage, disposes of debris and pathogens, alerts the adaptive immune system, and sets up repair. 2. The four cardinal signs of acute inflammation are redness, heat, swelling, and pain. 3. Chemicals cause vasodilation, to increase blood flow to the area, and increased permeability, which allows fluid containing clotting factors and antibodies to enter the tissues. 4. Soon after inflammation, the damaged site is invaded by neutrophils and macrophages. a. Injured cells produce leukocytosis-inducing factors that induce neutrophils to enter the blood from the bone marrow, increasing their number. b. Inflamed endothelial cells produce CAM proteins that mark the cell—a process called margination. c. Continued signaling causes diapedesis, in which neutrophils squeeze into the tissues between endothelial cells of the capillary walls. d. Inflammatory chemicals create chemical trails that encourage chemotaxis of neutrophils and other WBCs into the site of damage. D. Antimicrobial proteins enhance the innate defenses by attacking microorganisms directly or by hindering their ability to reproduce (pp. 771–773; Figs. 21.5–21.6; Table 21.2). 1. Interferons are small proteins produced by virally infected cells that help protect surrounding healthy cells (p. 771; Fig. 21.5; Table 21.2). 2. Complement refers to a group of about 20 plasma proteins that provide a major mechanism for destroying foreign pathogens in the body (pp. 771–773; Fig. 21.6; Table 21.2). a. Three pathways by which complement can be activated are: the classical pathway, involving antibodies to bind pathogens and complement; the lectin pathway, in which lectin proteins bind to sugars on the surface of the microorganism, and then bind to and activate complement; and the alternative pathway, triggered when complement factors interact on the surface of microorganisms. E. Fever, or an abnormally high body temperature, is a systemic response to microorganisms (p. 773; Table 21.2). 1. Pyrogens produced by leukocytes and macrophages act on the hypothalamus, causing a rise in body temperature. Part 2: Adaptive Defenses (pp. 773–796; Figs. 21.7–21.21; Tables 21.3–21.7) III. Antigens (pp. 773–774; Fig. 21.7) A. Aspects of the Adaptive Immune Response (pp. 773–774) 1. The adaptive defenses recognize and destroy the specific antigen that initiated the response. 2. The immune response is a systemic response; it is not limited to the initial infection site. 3. The immune system has memory; after an initial exposure the immune response is able to recognize the same antigen and mount a faster and stronger defensive attack. 4. Humoral immunity is provided by antibodies produced by lymphocytes present in the body’s “humors” or fluids. 5. Cellular immunity is based on direct attack of microorganisms by lymphocytes and has living cells, rather than free proteins, as its protective factor. B. Antigens are substances that can mobilize the immune system and provoke an immune response (pp. 773–774; Fig. 21.7). 1. Complete antigens are able to stimulate the proliferation of specific lymphocytes and antibodies and to react with the activated lymphocytes and produced antibodies. 2. Haptens are incomplete antigens that are not capable of stimulating the immune response, but if they interact with proteins of the body they may be recognized as potentially harmful. 3. Antigenic determinants are a specific part of an antigen that are immunogenic and bind to free antibodies or activated lymphocytes. 4. Self-antigens are your body’s antigens that are not antigenic to you, only to others, and are identified as “self” by MHC (major histocompatibility complex) proteins on the surface of cells. IV. Cells of the Adaptive Immune System: An Overview (pp. 774–778; Figs. 21.8– 21.10; Table 21.3) A. Lymphocytes originate in the bone marrow and, when released, become immunocompetent and self-tolerant in either the thymus (T cells) or the bone marrow (B cells) (pp. 774–777; Figs. 21.8–21.9). 1. The B lymphocyte selection process is not well understood: the T lymphocytes undergo selection for immunocompetence by going through two processes: a. Positive selection, which allows only T lymphocytes that recognize MHC proteins to survive. b. Negative selection, which selects for T cells that do not recognize self-MHC proteins. 2. Naive B and T cells are exported from the primary lymphoid organs, the thymus and bone marrow, and sent to colonize the secondary lymphoid organs, such as the spleen, lymph nodes. 3. Immune cells in lymphoid organs are well-placed to encounter antigens, so the first encounter between a lymphocyte and antigens usually occurs there. a. Antigen binding with a particular lymphocyte selects that lymphocyte for further development, a process called clonal selection. 4. Once activated by clonal selection, a lymphocyte divides, producing an entire group of lymphocytes with an identical ability to bind to a given antigen. a. Most members of the clone become effector cells that actively fight the infection. b. A few members of the clone become memory cells that will respond quickly to future encounters with this antigen. 5. Genes within lymphoid stem cells determine which foreign substances our immune system can attack and destroy by coding for a specific set of pieces that can be combined in different ways to form a variety of antigen receptor sites. V. Humoral Immune Response (pp. 778–784; Figs. 21.11–21.15; Table 21.4) A. The immunocompetent but naive B lymphocyte is activated when antigens bind to its surface receptors (pp. 778–779; Fig. 21.11). 1. Clonal selection is the process of the B cell growing and multiplying to form an army of cells that are capable of recognizing the same antigen. 2. Plasma cells are the antibody-secreting cells of the humoral response; most clones develop into plasma cells. 3. The clones that do not become plasma cells develop into memory cells. B. Immunological Memory (pp. 779–780; Fig. 21.12) 1. The primary immune response occurs on first exposure to a particular antigen, with a lag time of about 3–6 days. a. After mobilization, the antibody titer in the blood rises, peaking in about 10 days, and then declines to a low level. 2. The secondary immune response occurs when someone is reexposed to the same antigen, and is a faster, more prolonged, more effective response. a. Mobilization of B cells takes only a few hours and rises to a much higher peak concentration after only 2–3 days, producing antibodies with a much higher binding affinity for the antigen that may persist for weeks or months. C. Active and Passive Humoral Immunity (p. 780; Fig. 21.13) 1. Active immunity occurs when the body mounts an immune response to an antigen. a. Naturally acquired active immunity occurs when a person suffers through the symptoms of an infection. b. Artificially acquired active immunity occurs when a person is given a vaccine. 2. Passive immunity occurs when a person is given preformed antibodies. a. Naturally acquired passive immunity occurs when a mother’s antibodies enter fetal circulation. b. Artificially acquired passive immunity occurs when a person is given preformed antibodies that have been harvested from another person. D. Antibodies or immunoglobulins are proteins secreted by plasma cells in response to an antigen that are capable of binding to that antigen (pp. 780–784; Figs. 21.14–21.15; Table 21.4). 1. The basic antibody structure consists of four looping polypeptide chains linked together by disulfide bonds. 2. Antibodies are divided into five classes based on their structure: IgM, IgG, IgA, IgD, and IgE. 3. Antibody Targets and Functions a. Complement fixation and activation occurs when complement binds to antibodies attached to antigens and leads to lysis of the cell. b. Neutralization occurs when antibodies block specific sites on viruses or bacterial exotoxins, causing them to lose their toxic effects. c. Agglutination occurs when antibodies cross-link to antigens on cells, causing clumping. d. Precipitation occurs when soluble molecules are cross-linked into large complexes that settle out of solution. 4. Monoclonal antibodies are commercially prepared antibodies specific for a single antigenic determinant.