* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download Soil Nitrogen
Survey
Document related concepts
Transcript
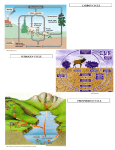
Soil Nitrogen Ammonia Ammonium Amino Acids Protein Nitrate Nitrogen Fixation Nitrogen is one of the major elements required for life. It is an essential for plant life because it stimulates above-ground growth, and produces the rich green color that is characteristic of a healthy plant. Although molecular nitrogen (N2) makes up 78% of the atmosphere, this form of nitrogen cannot be used by animals and most plants to make essential amino acids and proteins. This molecular nitrogen must first be “fixed” (combined with oxygen or hydrogen) to compounds such as ammonia (NH3) or nitrate (NO3-), or some other organic form of nitrogen. Some nitrogen fixation occurs by lightning and some by bluegreen algae, however, the bulk of nitrogen fixation is performed by bacteria living in the soil. One variety of nitrogen fixing bacteria lives free in the soil, while the other variety lives within the root nodules of legume plants (soybean, peanut, beans, clover, alfalfa). The decomposition of materials on the forest floor is also a source of nitrogen because ammonia or ammonium (NH4+) is produced in the process of decomposition. The movement of nitrogen from the atmosphere to inorganic forms such as ammonia and nitrate, followed by the incorporation of nitrogen into plant matter is represented by the nitrogen cycle, which is shown in the figure below. If soil is deficient in nitrogen, plants become spindly, stunted, and pale. The rate of plant growth is usually proportional to the rate at which nitrogen is supplied. However, an excess of nitrogen can damage plants just as overfertilizing the lawn can burn and damage the grass. Because nitrogen is an element essential to plant growth, but is most often found in relatively low quantities in forest soils, nitrogen, along with phosphorus, often becomes the nutrient that limits plant growth and therefore limits forest productivity. The ammonium ion can bind to clay particles to remain in the soil, however, the nitrate ion does not and is often washed or “leached” from the soil by water. Most of the nitrogen in soils will be found in the upper soil horizons and as a consequence, nitrogen can easily be depleted in soils when a disturbance occurs. If the soil lacks adequate amounts of organic matter, resulting in poor aggregate structure, porosity, and high levels of compaction, nitrogen will be leached (or washed) from the soil. Additionally, when the erosion process removes the upper soil horizons, including deposited organic matter, nitrogen sources are removed from the ecosystem. Procedure A – CHEMetrics Nitrate Test Kit Use the soil filtrate from the Soil pH procedure as the solution for analysis with the CHEMetrics Nitrate Test Kit. This kit gives results for nitrate levels under 5 ppm and can read as low as 0.1 ppm. The nitrate kit with 30 ampoules is $ 53.70, however, there is a educational kit with 10 ampoules for $13.20 (Although this seems like a better deal, two kits are required to achieve the same range as the larger kit. One kit measures 1-5 ppm, the other 0-1 ppm). There are also kits available at the same cost for ammonia and nitrite nitrogen. Procedure B – Test Kit Procedures for Nitrates and Ammonia Inorganic Nitrogen Test Kit Procedures *Reprinted from LaMotte Company Test Instructions Equipment and Materials (with LaMotte part numbers) Extraction Tubes 0704 Plastic Soil Measure Transfer Pipet 1 mL Pipet 0.5 g Spoon Spot Plate Ammonia Color Chart Nitrate Color Chart Nitrite Color Chart 0819 0364 0354 0698 0159 1302 1315 1310 Universal Extract Solution 5173 Ammonia Test Solution Nitrate Test Reagent #1 Nitrate Test Reagent #2 Nitrite Test Reagent #1 Nitrite Test Reagent #2 Nitrite Test Reagent #3 5103 5146 5147 5151 5152 5153 Extraction Procedure The following extraction procedure uses *Universal Extracting Solution to produce a single soil extract which is used in all of the three inorganic nitrogen tests, ammonia, nitrate, nitrite. 1. 2. 3. Fill the extraction tube to 14 mL line with extracting solution. Use the plastic soil measure to add two level measures of soil to the tube. Cap and shake for one minute. Using a piece of filter paper and plastic funnel, filter the soil solution. Catch the filtrate in the second extraction tube. Ammonia Nitrogen A fertile soil may be expected to give a low ammonia nitrogen test reading, unless there has been a recent application of nitrogenous fertilizer in forms other than nitrate. The rapid disappearance of ammonia after fertilizer application indicates the desired transformation of the ammonia to the more available nitrate compounds. In forest soils ammonia is the most abundant available form of nitrogen. If there is a satisfactory rate of nitrogen transformation, the humus layers of a forest soil will produce very high concentrations of ammonia nitrogen. 1. 2. Use a transfer pipet to transfer 4 drops of the general soil extract (filtrate) to one of the large depressions in the spot plate. Add one drop of *Ammonia Nitrogen Test Solution. Stir with a clean stirring rod. Allow to stand for one minute. 3. Compare the resulting color against the Ammonia Color Chart. The color chart expresses the results in relative values from very low to very high. Values can be converted to ppm according to the table below. Very low Low Medium High Very high 0–5 5 – 10 40 100 150 ppm ppm ppm ppm ppm Nitrate Nitrogen 1. 2. 3. 4. 5. Use a 1 mL pipet to transfer 1 mL of the general soil extract (filtrate) to one of the large depressions in the spot plate. Add 10 drops of *Nitrate Test Reagent #1 Use a .5 g spoon to add one level measure of *Nitrate Test Reagent #2 Stir thoroughly with a clean stirring rod. Allow to stand for five minutes. Match the sample color with the Nitrate Nitrogen Color Chart. The color chart gives nitrate values in lb/acre. Convert this value to ppm. 1 ppm = 2 lb/acre