* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download Risk Factors for Drug-Induced TdP
Pharmacognosy wikipedia , lookup
Neuropsychopharmacology wikipedia , lookup
Psychedelic therapy wikipedia , lookup
Neuropharmacology wikipedia , lookup
Pharmaceutical industry wikipedia , lookup
Pharmacokinetics wikipedia , lookup
Prescription costs wikipedia , lookup
Drug interaction wikipedia , lookup
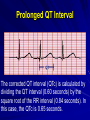
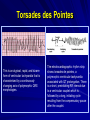
IATROGENIC SUDDEN DEATH 11th International Symposium Heart Failure & Co Reggia di Caserta; April 30, 2011; 12:05 P.M. Maria Rosa Costanzo, M.D., F.A.C.C, F.A.H.A Medical Director, Midwest Heart Specialists Heart Failure and Pulmonary Arterial Hypertension Programs Medical Director, Edward Hospital Center for Advanced Heart Failure Naperville, Illinois, U.S.A. Prolonged QT Interval The corrected QT interval (QTc) is calculated by dividing the QT interval (0.60 seconds) by the square root of the RR interval (0.84 seconds). In this case, the QTc is 0.65 seconds. Torsades des Pointes This is an atypical, rapid, and bizarre form of ventricular tachycardia that is characterized by a continuously changing axis of polymorphic QRS morphologies. The electrocardiographic rhythm strip shows torsades de pointes, a polymorphic ventricular tachycardia associated with QT prolongation. There is a short, preinitiating RR interval due to a ventricular couplet which is followed by a long, initiating cycle resulting from the compensatory pause after the couplet. The Ionic Basis of Ventricular Repolarization James AF et al. Prog Biophysics Molecular Biol 2007; 94: 265-319 Proposed Cellular Mechanism for the Development of TdP in the LQTS LQTS Intrinsic Heterogeneity Net Repolarizing Current ↓IKr, ↓IKs, ↑ICa, ↑late INa QT Prolonging drugs and ion channel mutations Prolongation of APD, Preferentially in M cells EAD-induced triggered beat Long QT ↑ Dispersion of refractoriness Torsade de Pointes (Reentry) Harvekamp W et al. EHJ 2000; 21: 1216-31 Terfenadine-Induced Brugada-Like Phenotype Di Diego JM et al. Circulation 2002; 106: 2004-11 Risk Stratification for ACA or SCD in LQTS Patients 14% Very High Risk (Secondary Prevention): Post-CPR or Spontaneous TdP High Risk 3% (Primary Prevention): Either one or more: QTc > 500msec Prior Syncope Low Risk: 0.5% QTc ≤ 500 msec and No Prior Syncope Moss AJ et al. Circulation 2000:101:616-23 QT Prolonging Drugs and Sex-Related Differences in TdP (http://www.torsades.org) Drug Drug Class Sex/Incidence of Tdp hERG/Ikr Blocker F>M Yes, mixed reaction Amiodarone Anti-Arrhythmic Arsenic trio-oxide Anti -cancer Bepridil Anti-anginal Cloroquine Anti-malarial Chlorpromazine Anti-psychotic/Anti-emetic F>M Yes Cisapride Gi stimulant F>M Yes Clarithromycin Antibiotic F>M Yes Disopyramide Anti-Arrhythmic F>M Yes Dofelitide Anti-Arrhythmic F>M Yes Domperidone Anti-nausea Yes Dropedirol Sedative/Anti-nausea Yes Erythromycin Antibiotic F>M Yes Halofantrine Anti-malarial F>M Yes Haloperidol Anti-psychotic F>M Yes, mixed reaction Ibutilide Anti-Arrhythmic F>M Yes Levomethadyl Opiate antagonist Mesoridazine Anti-psychotic Methadone Opiate agonist F>M Yes Pentamidine Anti-infective F>M Yes, trafficking Pimozide Anti-psychotic F>M Yes Procainamide Anti-Arrhythmic Quinidine Anti-Arrhythmic F>M Yes Sotalol Anti-Arrhythmic F>M Yes Sparfloxacin Antibiotic Yes Thioridazine Anti-psychotic Yes Yes, trafficking F>M Yes Yes ? Yes Yes Association between Effective Free Therapeutic Plasma Concentration and the Composite End Point of CA, SD, TdP, VT or VF 9.60 10 9 8 5.97 7 6 OR 5 4 ) 3 1.00 1.23 0.90 2 1 0 < 1/1000 1/1000-1/100 1/100-1/10 De Bruin ML et al. EHJ 2005; 26: 590-7 1/10/2001 >1 ETCP/IC50 Prolongation of the QT Interval, APD, and Transmural Gradient After Exposure to Representative Drugs in an Arterially Perfused Rabbit LV Wedge Preparation Liu T et al. Heart Rhythm 2006; 3: 948-56 Conditions Predisposing to TdP Cardiac Abnormalities – Ventricular Hypertrophy – Heart Failure – Previous anti-arrhythmic therapy Electrolytes and Metabolic Disorders – – – – – – Hypokalemia Hypmagnesemia Hypocalcemia Severe hypothyroidism Anorexia nervosa Hypo-/Hyperglycemia Bradyarrhythmias Sympathetic activity Female gender Transmural Action Potentials in the Rabbit LVH Renovascular Model Yan GX et al. Am J Physiol Circ Physiol 2001; 281: H1968-75 Risk Factors for Drug-Induced TdP Drug regimen – High drug doses or concentrations (except quinidine – Rapid IV infusion – Concurrent use of other drugs that can prolong the QT interval or that slow drug metabolism due to inhibition of hepatic cytochrome P450 enzymes (erythromycin, cimetidine, grapefruit juice Risk Factors for Drug-Induced TdP ECG abnormalities – Baseline QT prolongation or T wave lability – Development of marked QT prolongation, T wave lability , or T wave morphologic changes during therapy – Bradycardia which may be related to a fall in local extracellular [K], leading to enhanced drug-induced inhibition of Ikr – Congenital long QT syndrome or “silent” mutations in LQTS genes Risk Factors for Drug-Induced TdP Metabolic Factors – Electrolyte disturbances, such as hypokalemia, hypomagnesemia, hypocalcemia – Impaired hepatic and/or renal function Other – Underlying heart disease, particularly heart failure and LV hypertrophy – Recent conversion from AF – Female sex ESC-Proposed Algorithm for Evaluating QT Effects of Drugs in Development Critical Evaluation of the Expected Clinical Value of New Compound MOLECULE STRUCTURE Any similarities to compounds known to prolong ADP/QT? IN VITRO TESTS Cloned Channels Isolated cells/tissue Isolated heart Use model with which you have greatest experience Use reference compounds known to affect ADP/Qt Use appropriate experimental conditions (long cycle length, ↓K Include “major” metabolites ADP/QT↑ No Effect Reevaluate Abandon IN VIVO TESTS Proceed Use models applied in toxicology/safety pharmacology Perform serial ECG measurements QT↑ Reevaluate Abandon No Effect PHASE I/II CLINICAL TRIALS Proceed Use appropriate study design, adjust ECG recordings with plasma levels. timing should include “active metabolites, assess gender-related effects Schematic of Representative Design of Thorough QT Study Link M G et al. Circ Heart Fail 2010;3:547-555 Management of LQTS Acquired LQTS – Pharmachologic Magnesium Sulfate Isoproterenol Lidocaine Phenytoin Sodium bicarbonate (for quinidine-mediated arrhythmias) – Non-Pharmachologic Temporary Pacing (atrial or ventricular) Congenital LQTS – Pharmacologic Beta blockers Mexiletine – Nonpharmacologic Permanent dual chamber pacemaker Left cardiac sympathetic denervation (cardiothoracic sympathectomy) ICD Magnesium Sulfate First Line Therapy Effective for both treatment and prevention of LQTrelated ventricular ectopic beats or TdP Benefit occurs without QT shortening Benefit occurs in pts. with normal serum [Mg] at baseline Standard regimen: – 2 g IV bolus of 50% MgSo4 over 1-2 min followed in 15 min by another such bolus if required. – Some pts. receive 3-20 mg/min continuous infusion – Bolus dose in children 25-50 mg/Kg – No data on IV maintenance dosing in children Temporary Transvenous Overdrive Pacing Generally reserved for pts. with LQTrelated TdP unresponsive to IV Mg Pacing rates of ≈ 100 bpm ↓ dispersion of refractoriness and EADs development and may shorten surface QT, especially with bradycardia Many class IA and III antiarrhythmic drugs that cause TdP have “reverse use dependency” mediated in part by changes in the extracellular [K] changes Isoproterenol Initial dose 0.05-0.1 mcg/Kg/min in children and 2mcg/min in adults, then titrated to achieve HR of 100 bpm Can be used to ↑ sinus rate and ↓ QT Can be used as temporizing measure before pacing Other Acute Therapy Alkalinization of the Plasma: – Sodium bicarbonate – Useful when TdP is due to quinidine – Qud +OH- <-> QudOH IV K – May be useful in pts. with nl. [K] – 0.5 meq/Kg to a maximum of 40 meq ↑ plasma [K] by 0.7 meq/L, reverses QT prolongation and QT morphologic changes and ↓ QT dispersion – Effectiveness in preventing or reversing Tdp uncertain Chronic Management of Acquired LQTS Treatment of underlying cause – Discontinuation of offending drug – Correction of metabolic abnormalities Avoidance of drugs that prolong QT interval Nutritional rehabilitation in pts. with eating disorders PPM in pts. with chronic bradycardia or pausedependent TdP Thorough history and ECG screening of immediate family members because of occasional association with congenital LQTS Cumulative Probability of Death in Patients with LQTS with ACA or Recurrent Syncope Zareba W et al. J Cardiovasc Electrophysiol 2003; 14: 337-41 Diuretic Use and the Risk of Mortality in Patients with Left Ventricular Dysfunction Mortality Risk by Diuretic Use at Baseline Diuretic (n=2901) No Diuretic (n=3896) N Incidence N Incidence P value Death: all cause 1013 12.8 586 5.3 0.001 CV Death 903 11.4 510 4.6 0.001 Sudden Death 241 3.1 183 1.7 0.001 SOLVD database Cooper HA et al. Circulation. 1999; 100(12): 1311 Side Effects of Aldactone 25 Aldactone complications more frequent vs. trials VA (TX) 20 RALES 15 Dosing tends to be higher in the community 10 – RALES dose 12.5-25 mg/d D/C Rates G-Mastia 0 K > 6.0 5 Bozkurt et. al., JACC 2003;41:211 after RALES: RX Juurlink et al. NEJM 2004;351:543 after RALES:Death Juurlink et al. NEJM 2004;351:543 In-Hospital Outcomes Abraham WT etal. JACC 2005;46(1):57-64 Mortality Odd Ratios Pair-Wise Treatment Comparisons NES (n=4663) Vs MIL (n=1534) NES (n=4270) Vs DOB (n=3301) NES (n=4402) Vs NTG(n=5668) Unadjusted 0.53 (0.44–0.64)† 0.37 (0.32–0.44)† 1.64 (1.38–1.94)† Adjusted for covariatesa 0.59 (0.48–0.73)† 0.47 (0.39–0.56)† 0.95 (0.78–1.16)‡ Adjusted for covariates & propensity scoreb 0.59 (0.48–0.73)† 0.47 (0.39–0.56)† 0.94 (0.77–1.16)‡ Analysis* Hosmer-Lemeshow goodness-of-fit test not significant at 5% levels for the models adjusted for risk factors and/or propensity, except for covariateadjusted NTG vs. DOB comparison, where p 0.04. Area under the receiver operator curve 0.70 or higher. Because of multiple pair-wise comparisons, only p values 0.008 were considered significant using Bonferroni correction. *Patients taking both medications were excluded from †-p 0.005. ‡-p 0.58. §-p 0.021 for covariate adjustment and 0.027 for covariate and propensity score adjustment. aCovariates include age, gender, SBP, DBP, BUN, creatinine, sodium, heart rate, and dyspnea. each pair-wise analysis. bCovariates included in the propensity score by treatment comparison are: NES vs. DOB: SBP, sodium, BUN, creatinine, age, weight, LVEF, edema; NES vs. MIL: SBP, age, LVEF, dyspnea, weight; NTG vs. DOB: SBP, sodium, BUN, heart rate, LVEF, symptom duration; NTG vs. MIL: SBP, BUN, LVEF, symptom duration, dyspnea, QRS 120 ms, previous revascularization; NES vs. NTG: SBP, BUN, creatinine, LVEF, symptom duration, edema, previous HF, QRS 120 ms; DOB vs. MIL: SBP, age, hemoglobin, heart rate, dyspnea, VTF. Abraham WT etal. JACC 2005;46(1):57-64 Survival OPTIME-CHF: Etiology and Mortality ; Felker GM et al. J Am Coll Cardiol. 2003 Effect of Levosimendan on Mortality Days Following Randomization 5 14 31 90 Placebo Levosimendan Placebo Levosimendan 1 5 0 0 5 14 1 1 REVIVE II 12 35 20 45 REVIVE I 4 5 1 4 REVIVE I + II Circ.in press Placebo 1 6 16 40 Levosimendan 5 15 21 49 Use of Inotropes in HF Therapy Dobutamine is know to be associated with an increase in myocardial oxygen consumption, heart rate and risk of arrhythmias1,2 Milrinone produces tachycardia and other arrhythmias, and is limited by hypotension in many patients2,3 Stimulation of the B-adrenergic pathway has been linked to HF disease progression4 – MOA on heart for both Milrinone and Dobutamine – Rationale for use of B-blockers in chronic HF therapy Other observations, including RCTs, have shown worse outcomes with positive inotropic agents in the treatment of ADHF2,5,6 1. Burger AJ, etal. Am Heart J 2002;144:1102– 8 2. Monrad ES etal. Circulation 1986;73 Suppl III:III168 –74. 3. Cuffe ES etal. JAMA 2002;287:1541–7. 4. Sackner-Bernstein J, Mancini DM. JAMA1995;274:1462–7 5. Felker GM etal. J Am Coll Cardiol 2003;41:997–1003. 6. Felker GM, Oconner CM. Am Heart J 2001;142:393– 401. Conclusions The majority of iatrogenic sudden deaths are due to the effects of drugs on cardiac repolarization Drugs-induced QT prolongation is more common in women than in men The occurrence of iatrogenic sudden death is influenced by drug regimen, ECG abnormalities, cardiac and metabolic abnormalities The risk of individual patients must be carefully assessed Knowledge of the acute and chronic treatment of iatrogenic sudden death is lifesaving