* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download Lecture 1: Crystal structure
State of matter wikipedia , lookup
High-temperature superconductivity wikipedia , lookup
Nanochemistry wikipedia , lookup
Quasicrystal wikipedia , lookup
Electromigration wikipedia , lookup
Crystallographic defects in diamond wikipedia , lookup
History of metamaterials wikipedia , lookup
Dislocation wikipedia , lookup
Geometrical frustration wikipedia , lookup
X-ray crystallography wikipedia , lookup
Tight binding wikipedia , lookup
Low-energy electron diffraction wikipedia , lookup
Condensed matter physics wikipedia , lookup
Electronic band structure wikipedia , lookup
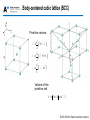
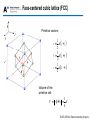
Lecture 1: Crystal structure OUTLINE • Crystal binding • Crystal structures • Miller indices ELEC-E3140 Semiconductor physics • Crystal binding • Crystal structures • Miller indices ELEC-E3140 Semiconductor physics Crystal binding • Ionic bond: one electron is transfered from one atom to the other • Covalent bond: Valence electrons are in common between neighboring atoms Na+ ClNa+ ClCl- ClNa+ C C Na+ Cl- Sodium chloride (ionic) C C C Diamond (covalent) ELEC-E3140 Semiconductor physics Covalent bonds in Silicon Electronic structure of Si: 1s22s22p63s23p2 4 valence electrons, 4 electrons missing to fill the outer shell Most electrons involved in the bonds are trapped in the bonds, and are not available for conduction. Pure silicon is a poor conductor But free carriers can be easily created by doping. ELEC-E3140 Semiconductor physics Covalent bonds in GaAs Electronic structure of Ga: 1s22s22p63s2p63d104s24p1 3 valence electrons, 5 electrons missing to fill the outer shell Electronic structure of As: 1s22s22p63s2p63d104s24p3 5 valence electrons, 3 electrons missing to fill the outer shell Ga As Ga As As Ga As Ga Ga As Ga As All valence electrons are used to create the covalent bonds ELEC-E3140 Semiconductor physics Heteropolar bonds In semiconductor crystals the bonds are often a mixture of covalent and ionic bonds Fractional ionic character IV-IV III-V Si 0.00 SiC 0.18 Ge 0.00 Fractional ionic character II-VI ZnO 0.62 ZnS 0.62 ZnSe 0.63 ZnTe 0.61 CdO 0.79 InP 0.42 InAs 0.36 InSb 0.32 CdS 0.69 GaAs 0.31 CdSe 0.70 GaSb 0.26 CdTe 0.67 ELEC-E3140 Semiconductor physics • Crystal binding • Crystal structures • Miller indices ELEC-E3140 Semiconductor physics Ordering in solid materials Single crystals – Atoms are ordered periodically i.e. they form a lattice – Translational symmetry, i.e. The crystal appears identical at several equivalent regions defined by a basic periodicity Polycrystals – Consist of several single crystals ordered randomly – Polycrystals are crystalline only locally ELEC-E3140 Semiconductor physics Ordering in solid materials Quasicrystals – Structure with symmetries but not translational invariance Amorphous – unordered atoms – No repeating crystal structure can be found in the material Quasicrystal pattern Amorphous SiO2 ELEC-E3140 Semiconductor physics Ordering in solid materials Amorphous SiO2 (glass) on top of crystalline silicon ELEC-E3140 Semiconductor physics Basic properties of crystal lattices • Crystal is formed from a lattice (Bravais lattice) and from a basis consisting of one or more atoms. • The basis is repeated with the symmetry of the Bravais lattice. • When travelling from a one lattice point to another, the surrounding atomic structure is the same (translational symmetry). • In addition to translational symmetry, the crystal can have rotational, mirror and inversion symmetries. ELEC-E3140 Semiconductor physics Basic properties of 2D crystal lattices Square lattice Hexagonal lattice Areas of crystal that can be repeated to form the full crystal are called (conventional) unit cells. The unit cell with the smallest area is called the primitive unit cell. The vectors defining (spanning) the unit cell are called primitive vectors. ELEC-E3140 Semiconductor physics Examples of 2D crystals Yksiatominen One atom basis kanta Kaksiatominen Two atom basis kanta ELEC-E3140 Semiconductor physics Primitive vectors Primitive vectors are not unambiguous – there can be several options (see red areas above). All the points in the lattice can be expressed by R = na 2 + mb 2 ; n, m = 0, ±1, ±2,.. ELEC-E3140 Semiconductor physics Wigner-Seitz primitive cell How to determine the Wigner-Seitz primitive cell: • Choose arbitrary atom (or basis) • Draw lines to neighboring atoms (or bases) • Draw the center normal to the line segment joining atoms (bases) • The area closest to the chosen atom (basis) and bounded by the normals is the Wigner-Seitz primitive cell • The Wigner-Seitz cell has the same symmetry as the crystal ELEC-E3140 Semiconductor physics Bravais lattices (2D) There are five different Bravais lattices in 2D. ELEC-E3140 Semiconductor physics Crystal lattice in 3D: unit cell • Unit cell = a region of a crystal defined by vectors a, b, c and angles α, β, and γ, which when translated by integral multiples of those vectors, reproduce a similar region of the crystal. Parallelopided indicating The basis vectors and angles. • Primitive unit cell = smallest unit cell in volume that can be defined for a given lattice, i.e. smallest periodically repeated pattern in the crystal. Most often the primitive vectors are defined as the sides of the primitive cell ELEC-E3140 Semiconductor physics Bravais lattices (3D) 14 lattices in 3D (= Bravais lattices) The most common semiconductors have a cubic lattice (a=b=c and α=β=γ=90°) – SC = Simple Cubic – BCC = Base Centered Cubic – FCC = Face Centered Cubic Simple Cubic Body-Centered Cubic Face-Centered Cubic ELEC-E3140 Semiconductor physics Bravais lattices (3D) ELEC-E3140 Semiconductor physics Simple cubic (SC) lattice Primitive vectors: a = dˆi b = dˆj c = dkˆ Volume of the primitive cell: V = a×b´c = d3 ELEC-E3140 Semiconductor physics Body-centered cubic lattice (BCC) Primitive vectors: a= d ˆ ˆ ˆ (i + j - k ) 2 b= d 2 c= ( -ˆi + ˆj + kˆ ) d ˆ ˆ ˆ (i - j + k ) 2 Volume of the primitive cell: V = a´b×c = d / 2 3 ELEC-E3140 Semiconductor physics Face-centered cubic lattice (FCC) Primitive vectors: 1 a = d ( i + j) 2 1 b = d (j+k) 2 1 c = d (k + i ) 2 Volume of the primitive cell: 1 V = a ×b´ c = d3 4 ELEC-E3140 Semiconductor physics Diamond structure Diamond structure = FCC lattice + 2 identical atoms in the primitive cell: (0,0,0) and (a/4, a/4, a/4) – Examples: Si, Ge and diamond Crystal viewer (diamond and Zinc blende structure): http://jas2.eng.buffalo.edu/applets/education/solid/unitCell/home.html ELEC-E3140 Semiconductor physics Zinc-blende structure Zinc-blende lattice = FCC lattice + 2 different atoms in the primitive cell – Examples: GaAs, InP, GaP, GaSb, InSb, ZnS, ZnSe, … (GaN, SiC and ZnO are difficult to manufacture in zinc-blende structure) ELEC-E3140 Semiconductor physics Hexagonal lattice Simple hexagonal lattice (the blue atoms): Primitive vectors: a = a ˆi b= ( a ˆ i+ 2 3 ˆj ) c = c kˆ ELEC-E3140 Semiconductor physics Hexagonal close packed lattice Hexagonal close packed structure (HCP) consists of two simple hexagonal lattices translated by a vector a 3+b 3+c 2 ELEC-E3140 Semiconductor physics Wurtzite structure Two HCP lattices translated by a vector (in orange) with different atoms in each lattice. Examples: GaN, SiC, ZnO, AlN ELEC-E3140 Semiconductor physics Wurtzite and zinc blende structures Difference between wurtzite and zinc blende structures: ELEC-E3140 Semiconductor physics Crystal lattice: parameters • Lattice constant = the length of a side in a cubic lattice • Coordination number = the number of nearest neighbor lattice sites. For example in BCC lattice it’s 8 and in FCC it’s 12. • Nearest neighbor distance = distance between 1 neighboring atoms, for FCC it’s 2 a 2 • Packing fraction (assuming spherical atomic shells)= (volume of each sphere x number of spheres ) / total volume of the unit cell ELEC-E3140 Semiconductor physics Lattice constants ELEC-E3140 Semiconductor physics • Crystal binding • Crystal structures • Miller indices (next week) ELEC-E3140 Semiconductor physics